OTTO HEINRICH WARBURG
OTTO HEINRICH WARBURG 1883-1970
Elected For. Mem. R.S. 1934
Otto Warburg died on 1 August 1970, after avery short illness. In the course of a career devoted entirely to research and extending over 60 years he made an exceptionally large number of highly original and far-reaching contributions to cell biology and biochemistry. In an age which has produced many outstanding scientists, he stands out as one of the great pioneers of contemporary biology. Lewis and Randall (31)*, in the preface to their dynamics, liken the edifice of science to a cathedral built by the efforts of a few architects and many workers. In this sense Warburg belongs to the small band of real architects of his generation.
He was born on 8 October 1883, in Freiburg im Breisgau where his father, Emil Warburg, was at that time Professor of Physics at the University. Emil Warburg (1846-1931) was one of the leading physicists of his time who made many classical contributions to his subject and formed a large school. An appreciation of his achievements and personality was published by James Franck (18), one of his students.
Family and early days
The Warburg family (8, 37, 41, 44, 45, 46) traces its origin from one Simon Jacob, who in the 16th century lived in the town of Warburg in Westphalia near Cassel. The story goes that he was a banker to the Margrave of Hesse-Cassel and that he was pressed by his sovereign to abandon his Jewish faith to be baptized. He refused and migrated to the small town of Altona near Hamburg where he adopted the surname of Warburg. The local rulers of that area of Germany—the Grafen von Holstein and, after 1640, the Danish crown—were somewhat exceptional in respect to their liberal attitude in matters of creed, trade and commerce. This attracted refugees from other parts of Germany and from Holland. Otto was the tenth direct descendant of Simon Jacob Warburg all of whom, except Otto, were born and lived in Altona where the family over many generations played an important part in local affairs. Descendants of Simon Jacob Warburg include many who distinguished themselves in scholarship, business, the arts, public service and as benefactors; they spread over many countries.
* Figures given in round brackets refer to the list of references. Figures given in square brackets refer to the Bibliography.
Biographical Memoirs
Hie banking house of M. M. Warburg in Hamburg was established in 1798. The art historian, Aby Warburg (1866-1929), was one of the pioneers in his subject. He assembled a unique art history library, financed by his banker brothers. After the advent of the Hitler regime, it was fortunately possible to transfer this with its staff to London where it formed the nucleus of the Warburg Institute of London University. Sir Siegmund Warburg of London, the distinguished banker, is another member of the family. Sir Oscar Warburg (1876-1937) was a wrangler at Cambridge, a member of the London County Council from 1910-1931 and its Chairman in 1925-26. He was a successful horticulturist, business man and public servant. The publisher, Fredric J. Warburg, of Seeker and Warburg, also belongs to this family. Another member was the botanist and Zionist leader, Otto Warburg (1859-1938), who distinguished himself in the field of tropical agriculture.
Otto Warburg’s mother was Elisabeth Gartner. She is spoken of as a woman of great vitality and wit. Otto himself thought that essential traits of his personality stemmed from his mother and her family. She was of South German stock; her family and ancestry included several people who have distinguished themselves as administrators, lawyers and soldiers. A brother was killed in action in World War I serving as a General.
Otto had no brothers but three sisters. In 1895, when Otto was in his 12th year, the Warburg family moved from Freiburg to Berlin. At the Friedrichs-Werder Gymnasium he was a good pupil though he was occasion- ally prone to mischievous pranks as the following communication from the school to his parents of 14.3.1896 indicates:
‘The pupil Warburg (lower fourth) has repeatedly taken part in gross misconduct and he has encouraged fellow pupils to join in. When inter viewed he exhibited a regrettable lack of truthfulness. This is doubly regrettable in view of his considerable talents and accomplishments and it is urgently desired that these bad traits are energetically acted against at home’.
This trait was certainly corrected: in adult life he became fanatically concerned with establishing truth, a point which he often expressed in his polemic passages and general comments.
The parental home in Berlin became a stimulating cultural centre which brought young Otto into close contact with leading Berlin academics as well as with artists, for his father was an accomplished pianist who played chamber music in his home. Otto learned the violin but gave it up when he realized that he could not attain his father’s standard of accomplishment. He main tained, however, a life-long love for, and appreciation of, music.
Through his father, Otto got to know Emil Fischer (1852-1919), the leading organic chemist of his day, Walther Nernst (1864-1941), the leading physical chemist and physicist and Jacobus Henricus van’t Hoff (1852-1911), the pioneer physical chemist, and Theodor Wilhelm Engelmann (1843-1909), the physiologist from whom he first heard something about photosynthesis. Max Planck and Albert Einstein were other frequent contacts.
In 1901, Warburg began his studies of chemistry at the University of Freiburg. As was customary in central Europe, he later moved to another university, Berlin, and completed his studies with a doctoral thesis under Emil Fischer in 1906. The main subject matter of this work was the prepara tion of optically active peptides and the study of their enzymic hydrolysis. Fischer himself, though head of a large institute with many teaching com mitments, spent most of his working time at the bench, side by side with his research students. He set an example in his style of working, his high stand ards of reliability and personal integrity on which W arburg modelled himself throughout his life.
Pre World War I period
Already in his student days, Warburg was exceptionally ambitious. It was his confessed aim to do better than his father—and by all ordinary academic standards his father had done extremely well, by reaching the top positions available to physicists in Imperial Germany: when Emil Warburg was appointed to the Chair of Physics at the University of Freiburg he was 30. Nineteen years later he was transferred to the Physics Chair at the University of Berlin, the most coveted post in the subject. Otto’s ambition, however, did not aim at top posts (which though giving high standing and power, con sumed too much time and energy on matters of academic routine), but at great discoveries. Very early, before he graduated, he fancied making a major contribution to the cancer problem, especially to the cure of cancer. Although he did not begin to work on cancer directly until 1922, much of his earlier work appears to be in retrospect a preparation for a fundamental attack on cancer.
It was his interest in medical problems which prompted him, after the completion of his doctoral thesis, to study medicine in Heidelberg. In his spare time he worked in the laboratory of the Department of Internal Medicine. Under Ludolf Krehl—a distinguished physician and the author of a standard book Pathological Physiology—this was a great centre of medical research where the relevance of the basic sciences to clinical medicine was appreciated. He obtained his M.D. degree in 1911.
Warburg remained at Heidelberg until 1914, spending occasional periods at the Naples Zoological Station. During the eight years at Heidelberg, he published some 30 major papers. His research students and collaborators during this period included Otto Meyerhof and Julian Huxley.
His very first major independent piece of work, published in 1908, was concerned with the energetics of growth. It dealt with the changes in the oxygen consumption which take place when a sea urchin egg, after fertilization, begins to grow. These experiments led to the important discovery that on fertilization the rate of oxygen consumption rises up to 6-fold [6, 8, 10].
Already at this stage, Warburg showed exceptional skill in selecting the right kind of material and in perfecting experimental techniques. He chose the sea urchin egg because the mass of live matter is large in relation to the yolk mass, and the development of the fertilized egg is very rapid so that, as he put it, much happens in a short time. His reasoning was that chemical work is done when living matter arises in the course of growth and therefore the rate of the energy supply must rise. Experiments of previous authors on this problem had been inconclusive. By improving the titrimetric method of oxygen determination according to Winkler and carefully checking possible sources of errors, his results were convincing and established him as an out standing investigator.
The link between this work and the later investigations on cancer is obvious: when a normal cell becomes cancerous, it grows excessively, and in 1922 Warburg set out to test whether cancer cells have an increased oxygen consumption (see later).
In his subsequent work, Warburg was primarily concerned with funda mental aspects of cell respiration. It was his aim to unravel the nature of the catalysts in living matter which enable molecular oxygen to oxidize sub stances in near-neutral solution and at biological temperatures that are completely stable towards oxygen. He also appreciated early that a key problem of biological energy transformations is the question of how the free energy made available by combustion is utilized by muscle and other tissues. Fick (17) had made it clear in 1893 that living cells cannot be heat engines, i.e. engines in which heat is an intermediary stage of energy.
Warburg’s approach was guided by the conviction that all processes in living matter obey the laws of physics and chemistry, a view now taken for granted but not generally accepted when he entered the field. In 1897 Buch ner had succeeded in extracting yeast fermentation from the cell and thus obtaining fermentation in solution. At the time it was held that chemical processes cannot be studied in the solid or semi-solid state (in which they take place in vivo) and that it was necessary, as a first step, to bring them into solution and secondly to isolate the components and prepare them in a pure state. When attempting to solubilize ‘respiration’ i.e. the oxidation of normal cell constituents by molecular oxygen, Warburg found that the main oxidative processes were always attached to insoluble particles, to what he called ‘structures’. In 1913, he described ‘grana’ from liver cells as the struc tures to which respiration is bound [33]. We now know that these ‘grana’ were mitochondria, but it took another 30 years before, in the hands of Claude, Hogeboom, Hotchkiss and Schneider, the morphological structure described in the 1890s as mitochondria were shown to be identical with Warburg’s grana and constituted the ‘power plant’ of the cell (10). To this day, the key reactions of molecular oxygen are virtually inseparable from in soluble components. They are, as Warburg expressed it, ‘structure-bound’ and as we now say, ‘membrane-bound’. Warburg made many ingenious efforts to characterize the properties of the structure-bound oxidations.
He studied in particular how they can be influenced by extraneous substances, hoping that by such effects information about the nature of the catalysts of respiration could be discovered. He established that many substances such as alcohol, nitriles, or urethanes inhibit oxidations according to their surface activity and he concluded that narcotics—substances which are chemically relatively indifferent and inhibit respiration reversibly—interfere on account of their adsorption to the surface of the oxidizing structures preventing access of substrates to the enzyme. An exception where surface activity was clearly irrelevant was the action of cyanide, a powerful inhibitor of biological oxida tions. Since cyanide readily reacts with heavy metals, Warburg rightly concluded that heavy metal catalysts must be involved in cell respiration. He demonstrated the presence of iron in biological material and by ‘model’ experiments—the acceleration of the oxidation of tartrate, lecithine, linolenic acid, cysteine and aldehydes by iron salts [35]—he supported the hypothesis that the heavy metal concerned is an iron compound.
Warburg summarized the work carried out between 1906 and 1914 and its general implications in a comprehensive review [40].
A later generation of scientists occasionally has difficulties in appreciating how Warburg arrived at the concept of ‘structure-bound’processes in the cell. Thus in his Nobel Lecture, Luis Leloir (30) wrote in 1971: ‘In 1936 we managed to prepare a cell free system which was active when suitably supplemented and this was a novel result since the process of oxidation was believed to require the integrity of the cells. I suppose theyoung generation of biochemists finds it dijficult to understand many o f the things which we believed at that time’ (my italics).
This comment is somewhat misleading because the statement that oxidations in living cells require the integrity of the cell structure was not a matter of belief; it was purely descriptive. It expressed the fact that the capacity to oxidize was lost (or largely lost) when the organised structure of cells is destroyed. It was only in the later 1930s that it became clear why the oxidative capacity (and other functions) were lost, namely because of dilution, or loss of, soluble co-factors, such as the adenine- and pyridine nucleotides. The oxidative capacity was also lost in the first instance in Leloir’s experiments when he prepared the liver extract but it was restored by the addition of AMP, phosphate, cytochrome C and fumarate.
In 1913, at the age of 30, Warburg was appointed ‘Member’ of the Kaiser Wilhelm Gesellschaft to take effect on 1 April 1914. This was an appoint ment as Head of research department which gave him complete freedom in the choice of his research subject and involved no teaching or administrative responsibilities whatsoever. The main movers in bringing about this appoint ment were Theodor Boveri, the biologist, and his former teacher, Emil Fischer. Fischer described the attitude of the Kaiser Wilhelm Gesellschaft as follows when in 1911 he (successfully) persuaded Richard Willstatter to accept a similar post: ‘You will be completely independent. No one will ever trouble you. No one will ever interfere. You may walk in the woods for a few years or, if you like, you may ponder over something beautiful’ (49). On the whole, this policy (which rested on extreme care and competence in selecting the right people) has paid magnificent dividends: Warburg, Einstein, Haber, Hahn, Meitner, Correns, Goldschmidt, Polanyi and many others were ‘members’ who made the fullest use of the opportunities.
While Warburg’s laboratory was being completed and equipped, he worked in the laboratory of Walther Nernst on oxidation-reduction potentials in living systems, but this work was interrupted by the outbreak of World War I on 1August 1914.
War service
Soon after the outbreak of the war, Warburg volunteered for service and applied to what was looked upon as a crack cavalry regiment, the Horse Guards (‘Garde Ulanen’) at Potsdam. He was accepted and soon saw service in the front lines. He rose to the rank of Lieutenant, was wounded in action and was decorated with the Iron Cross, First Class.
In March 1918 he received a letter from Albert Einstein (seven months before the end of fighting) which is characteristic of both Einstein and of Warburg.
In order to appreciate some points of the letter it should be borne in mind that Einstein for many years had been a colleague and friend of Emil Warburg, Otto Warburg’s father. It was Emil Warburg who in the 1910s had provided the first experimental proof of Einstein’s prediction of the law of the photochemical equivalence. Emil Warburg and Einstein also had frequent social contacts because they played chamber music together. So Einstein had met Otto Warburg on many occasions in his parents’ home before the letter was written (translated from German).
‘Dear Colleague,
‘You will be surprised to receive a letter from me because up to now we have walked round each other, without actually getting to know each other. I even fear that by this letter I might arouse something like displeasure; but I must write.
‘I gather that you are one of the most able and most promising younger physiologists in Germany and that the representation of your special subject here is rather mediocre. I also gather that you are on active service in a very dangerous position so that your life continuously hangs on a thread: now for a moment please slip out of your skin and into that of another clear-eyed being and ask yourself: Is this not madness ? Can your place out there not be taken by any average man; Is it not important to prevent the loss of valuable men in that bloody struggle? You know this well and must agree with me. Yesterday I spoke to Professor Krauss who entirely shares my opinion and is also willing to make arrangements for you to be claimed for other work.
‘I therefore entreat you, as a consequence of what I have said, that you may assist us in our endeavours to safeguard your life. I beg you to send me, after a few hours of serious heart-searching, a few lines so that we may know here that our efforts will not fail on account of your attitude.
‘In the anxious hope that in this matter, as an exception, reason will for once prevail, I am with cordial greetings,
Yours sincerely,
A. EINSTEIN’
The letter illustrates the very high esteem in which Otto Warburg was held as a young scientist; he was, after all, not yet 31 when the war had started. It also indicates that he must have had the reputation of being strong- willed and not easily diverted from his views. In style and substance the letter is also typical of Einstein. It shows his humanity and his deep concern for younger people. There are many other instances where he troubled himself to intervene in order to help, as illustrated by the Einstein-Born (16) correspondence.
Incidentally, Einstein’s comment on the rather mediocre state of German physiology and biochemistry was perfectly fair, considering that his assess ment must have been based on the exceptionally high standing of the exact sciences in Germany at that time, distinguished by such names as Planck, Nernst, Born, von Laue, Wien, Emil Fischer, von Baeyer, Willstatter, Wieland, Ostwald, Wallach, Hans Fischer, Haber, Hilbert and Klein.
Warburg accepted Einstein’s proposal because, as he said, the war had been lost in any case, and he returned to Berlin soon afterwards. Perhaps Einstein’s intervention saved his life.
On his war service in World War I Warburg himself commented in a filmed interview recorded in 1966 by the Institut für den Wissenschaftlichen Film of Gottingen:
‘The only interruption in my research during the past 53 years was four years in World War I. I do not regret this interruption. In one of the finest uniforms of the old Prussian Army I rode many patrols in advance of the front line during the early advances in Russia. Later when the war of move ment had ended I was Orderly Officer to several of our great army com manders. In the course of this I got to know the realities of life which had escaped me in the laboratory. I learned to handle people; I learned to obey and to command. I was taught that one must be more than one appears to be’.
Post World War I period General aspects
After his return to civil life Warburg’s laboratory occupied a section of the top floor of the Kaiser Wilhelm Institut fur Biologie in Berlin Dahlem until 1931. It was not a large laboratory, providing initially benches for about six people, and after the transfer of the animal quarters in 1927 there were about ten places. Equipment for physical and chemical work was excellent. Throughout his life it was Warburg’s policy to keep the number of research workers low and to use a relatively large fraction of his financial resources for technical equipment. His long term collaborators throughout were technicians who in the 1920s occupied four of the few places—E. Negelein, F. Kubowitz, E. Haas and W. Christian, to be joined later by W. Liittgens. He recruited these technicians from skilled mechanics trained in the work shops of engineering firms. Their asset was that they knew how to deal with physical instruments, and Warburg trained them in chemistry himself. Many of the technicians, like those mentioned above, became well-known through their publications. Technicians whom he recruited after World War II (when he had to start from scratch) included G. Krippahl, K. Gawehn, H.-S. Gewitz, W. Vbiker, A. Lehmann, K. Jetschmann, S. Lorenz, A.-W. Geissler, W. Schroder, H. Klotzsch, EI. Geleick and H.-W. Gattung. Warburg found technicians ideal collaborators because they were content with their status and, unlike academics, were not prone to focus their interests on promotion and careers: they never troubled him with requests for testimonials and for support in obtaining better posts. He treated them well, and at the same time firmly. Their salaries were appropriate to their long working hours. They were devoted to him and stayed over many years.
Postdoctoral collaborators included H. Gaffron, IE. A. Krebs, W. Kempner, W. Cremer, H. Theorell, E. G. Ball, C. S. French, J. N. Davidson, Th. Bucher and D. Burk. The total number of research workers in his laboratory at any one time was usually no more than 10-12 of whom perhaps one or two were academics. This figure refers to the time after his move to a new In stitute in 1931.
In 1929 Warburg gave a review lecture at Baltimore entitled ‘Enzyme action and biological oxidation’. He recorded [435] that after this lecture the Rockefeller Foundation offered support for his research. He suggested the building of a small institute for cell physiology, as well as the foundation of a larger institute for physics under the direction of Max von Laue. He had in mind collaboration with physicists especially in the field of radiation, the application of which to biochemical problems had been a major source of his recent successes. Within six months the Rockefeller Foundation provided 635 000 marks for the purchase of the land required for the two institutes, 600 000 marks for the building and equipment of the institute for cell physiology and about 1.5 million marks for an institute of physics, all under the wing of the Kaiser Wilhelm Gesellschaft.
The new institute—the Kaiser Wilhelm Institut fur Zellphysiologie—was ready for occupation late in 1931. Warburg chose as a model for its outer style the fine 18th century rococo manor house at Grosskreutz near Potsdam, owned by the von der Marwitz family. The unusually attractive building provided all the space he needed, including facilities for large-scale chemical operations which were necessary for the initial steps of enzyme isolation. In 1943 air attacks made life in Berlin dangerous and Warburg with his staff and equipment moved to the Liebenberg estate, about thirty miles north of Berlin which the owner, Prince Eulenberg, a Prussian nobleman, placed at his disposal. Here work continued undisturbed until 1945 when the advancing Russians occupied this area and removed all the equipment from the laboratory. It has never been clarified who was responsible for this action but afterwards the Russian Commander-in-Chief, Marshall Zhukov, invited Warburg to see him and told him, in the name of the Russian Government, that the dismantling of his laboratory had been an error. The Marshall issued orders to return the apparatus and books but, alas, they could not be traced.
The Dahlem Institute did not suffer damage during the war. It was located in the American Sector of Berlin and for over four years it was used by the American forces as the seat of their Berlin High Command. They evacuated the building in 1949 and with the help from many quarters it was, in Warburg’s own words, ‘reconverted into the Kaiser Wilhelm Institut fur Zellphysiologie’ [435]. It was ceremonially re-opened on 8 May 1950, by General Maxwell D. Taylor, the Commandant of the American Sector of Berlin and later Chief Military Adviser of President John F. Kennedy. In 1953 the Institute was renamed Max Planck Institut fur Zellphysiologie. Thus for five years Warburg was unable to carry out experimental work but during this period he wrote two books, [506, 507] in which he summar ized his earlier work and discussed its significance, especially in relation to other investigators’ work. In 1948/49 he spent several months in the United States, at Urbana, Bethesda and Woods Hole.
Within a few years of Warburg’s return from World War I, three main lines of investigations emerged—photosynthesis, cancer and the chemical nature of the enzymes responsible for biological energy transformations i.e. of oxidations and reductions. These three subjects (in many ways interlinked) with their numerous ramifications occupied him throughout his life from 1919 to 1970. In all three he greatly advanced the methodology and made funda mental discoveries.
Advances in methodology Manometry
In his early studies Warburg had used titrimetric methods for measuring the oxygen uptake of sea urchin eggs and red blood cells but these procedures were cumbersome and not very sensitive. Having seen the Haldane-Barcroft ‘blood gas manometer’ at a brief visit to Barcroft’s laboratory at Cambridge, he began to use this instrument in 1910 when he referred to it as the ‘beautiful blood gas apparatus of Haldane—Barcroft’ [11]. This differed from earlier manometers in that a special device kept the volume of the gas space constant so that the pressure was the only variable when a gas was formed or removed at constant temperature. The originators had used the apparatus for measuring the quantities of oxygen bound to haemoglobin or the carbon dioxide content of blood. Warburg adapted it for the measurement of rates of gas exchanges. For this purpose it was necessary to maintain gas equilibrium between the liquid and the gas phase in the manometer vessel and this was achieved by constant shaking of the vessels in a water bath [57, 64, 77].
In many situations manometry proved far more accurate and far more convenient than the classical methods of gas analysis. It was applicable not only to processes in which gasses directly participate, as in cell respiration, photosynthesis and alcoholic fermentation, but also to reactions (such as the lactic acid production by animal tissues) which can be coupled with a gas- producing reaction by allowing it to take place in the presence of bicarbonate, the acid produced leading to the liberation of an equivalent amount of COa.
A special advantage of the manometric technique, as developed by War burg, and as opposed to other manometric or gasometric methods (for instance those of Van Slyke and of Haldane) is the fact that it measures increments directly whilst the other analytic procedures measure differences. Hence the presence of any amounts of, say, 0 2 or C 0 2 does not affect the accuracy of the measurements.
The theory and practice of manometry were perfected in the 1920s, but important further elaborations were added in the 1950s [508]. Mano metry was the key factor in the discovery of the lactic acid fermentation of cancer tissue, in much of the work on cell respiration, fermentation and photosynthesis, as well as in the identification of the iron porphyrin structure of the oxygen-transferring enzyme of cell respiration. The manometric method also proved exceedingly valuable as an analytical tool for the quantitative determination of small amounts of, for example, bicarbonate, urea, succinic acid, amino acids (by decarboxylation reactions) and purine bases.
A 600-page monograph Manometrische Methoden edited by A. Kleinzeller in 1966 describing the scope of manometry, including over 100 analytical procedures, is indicative of the value and range of the methods which Warburg initiated (27).
Spectrophotometry
In the 1940s, some aspects of manometry began to be superseded by spectro-photometric techniques, but this was again the result of Warburg’s fundamental discoveries and methodological ingenuity. It was Warburg who developed from 1928 onwards the use of the photoelectric cell and the provision of suitable sources of monochromatic light, in the first instance for his measurement of the action spectrum of the ‘0 2-transferring enzyme of respiration’ [129, 140]. Much later, in the 1940s, these principles were in corporated into the commercial spectrophotometers, the first to be marketed being that of the Beckman company. It was Warburg who discovered the absorption of reduced pyridine nucleotides at 340 nm [253], and who used this property as the basis of a large number of ‘optical tests’ for measuring reaction rates and quantities of metabolities. He furthermore established the principle that the extinction of reduced pyridine nucleotide at 340 nm can be used for the measurement of reactions and substances which are not directly involved in oxido-reductions. Coupling reactions can establish a link with dehydrogenase systems. Thus aldolase activity can be determined by coupling with glyceraldehyde-phosphate dehydrogenase so that a product of the aldolase reaction undergoes dehydrogenation. The optical tests were also essential tools in the purification and eventual crystallization of numerous enzymes. Requiring minute quantities of material and usually taking merely minutes to perform, they indicate in which fraction the active enzyme protein is contained.
Tissue slice technique
The use of tissue slices was developed by Warburg in the first instance for the measurement of the respiration of cancer cells because he wanted to test whether the oxygen uptake increases when cells begin to grow [57]. The object of slices was to have a sufficiently thin piece of tissue where diffusion from and to the suspending medium adequately supplies nutrients and removes waste products, a function normally effected by the blood circulation. Slices less than 0.5 mm thick are shaken in a saline medium resembling blood serum in respect to inorganic constituents, or in serum itself. Warburg developed the mathematical theory necessary for the calculation of the maximum thickness permissible to saturate the tissue with oxygen; this value depends on the rate of oxygen consumption, on the rate of diffusion within the tissue and on the concentration of Oa in the medium. Slices usually contain 100-150 layers of cells, and as only one cell on each side is cut and directiy damaged by the razor, the structure of the majority of cells is expected to remain intact. Intactness of cell structure was of importance because Warburg’s earlier work (already mentioned) had shown that the energy giving reactions are lost when the cell structure is disrupted. Thus it was necessary to devise a method for measuring the energy giving processes without mechanical destruction of the tissue and without addition of anti septics, points which the earlier investigators did not adequately appreciate. It is true it has since become possible to restore the energy giving reactions of homogenised tissues, but only by the addition of co-factors, and the rates observed in such preparations reflect the vivo rates much less reliably than those found in slices.
It is a decisive advantage of the slice technique that samples of the same organ can be incubated in parallel under a variety of precisely controlled conditions. In the case of most tissues, 50 to 100 mg fresh weight are sufficient for accurate measurements of oxygen consumption and lactate production. An alternative and already well established method was the perfusion of the isolated intact organs, of which Warburg had personal experience [13]. This method was applicable to organs like liver, kidney or heart from larger animals, which have a clear-cut vascular system through which they could be perfused. Cancer tissue, as a rule, has an irregular blood supply and there fore does not lend itself to perfusion experiments. Moreover, the perfusion technique does not allow parallel experiments to be carried out on the same tissue, and it could not (yet) be applied to small animals. It was therefore cumbersome and expensive.
The slice technique was originally designed to study the energy giving processes. Later it became clear that slices also perform many biosynthetic reactions and they proved an invaluable material in the study of inter mediary metabolism, degradative and biosynthetic, and also in the study of the mechanisms responsible for the transport of substances into and out of tissues. The tissue slice technique thus proved of great importance generally to the study of metabolic processes.
Cancer, tissue metabolism and the Pasteur effect
The cancer problem, as already mentioned, began to occupy Warburg’s mind in his student days when he became aware of the ravages of cancer and of the great limitations of successful treatment. His approach was to be a fundamental one—an attempt to find out what biochemical changes take place in a tissue when a normal cell (the growth of which is controlled) becomes a cancer cell (the growth of which is unrestricted). Does the meta bolism of cancer cells differ qualitatively from that of normal tissues? The question had been asked before but the earlier attempts to find an answer, in Warburg’s view, suffered from both conceptional and experimental short comings. If the abnormal growth rate had to be explained, he argued, one would have to consider in the first instance the reactions which provide energy for growth because without energy, growth cannot take place. He had in mind his earlier discovery that the respiration of the sea-urchin egg can increase up to 6-fold when the egg is fertilized and begins to grow, and he therefore set out in 1923 to measure the rates of respiration of a transplant able cancer—the Flexner-Jobling rat carcinoma—using the tissue slice technique developed for this purpose [58, 66, 504]. The results were clear- cut. Firstly, the rate of oxygen consumption of the cancer cells did not differ from that of a variety of normal cells. Secondly, cancer cells readily form lactate from glucose to an extent which far exceeds the rates of liver, kidney, pancreas and submaxillary gland. The rate was high enough to make a significant contribution to the energy supply of the tissue. These findings were soon confirmed for other types of neoplastic cells, including human cancers. A special feature of the high rate o f ‘glycolysis’ of cancer cells was its occurrence in the presence of oxygen. It had already been known before Warburg that many tissues, e.g. muscle, can form lactate from carbohydrate in the absence of oxygen.
Warburg asked himself at once whether the aerobic glycolysis is specific for neoplastic cells and he therefore examined systematically many tissues for their capacity to glycolyse aerobically and anaerobically. This led to the discovery that all animal tissues that are metabolically active glycolyse anaerobically and that the great majority of these do not glycolyse aerobically. The main exception was the retina of warm-blooded animals, the aerobic glycolysis of which was even faster than that of cancer tissue. Furthermore he found that some tissues, for example the haemopoietic bone marrow cells, gradually develop an aerobic glycolysis when they are exposed to un- physiological conditions in vitro. This he took to indicate that the aerobic glycolysis of the retina, a very delicate tissue, might be an vitro artefact.
Warburg related these discoveries to analogous observations which Pasteur had made 60 years earlier in micro-organisms. Pasteur established that the rates of fermentation are generally high anaerobically, but low aerobically. It was in fact Pasteur who coined the terms aerobic and anaerobic. So Warburg reached the conclusion that cancer cells differ from non-cancer cells, including growing embryonic cells, by their failure to suppress glycolysis in the presence of oxygen.
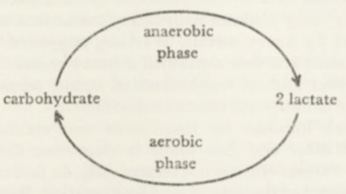
By this time, Meyerhof had already established that in muscle there is a regular relationship between the rate of respiration and the suppression of glycolysis: usually the consumption of one molecule of oxygen prevents the formation of the two molecules of lactate. This meant that the effect of oxygen cannot be explained (as previous investigators had thought) by a complete oxidation of lactate. Meyerhof suggested that many findings agreed with the assumption that the enzymes forming lactate also function aerobically but that the lactate formed is resynthesized to carbohydrate at the expense of the energy provided by respiration. He depicted the carbohydrate metabolism of muscle by this diagram:
He calculated that sufficient energy would become available from the consumption of one molecule of oxygen to bring about a resynthesis of carbohydrate from two molecules of lactate.
Having discovered the high aerobic glycolysis of cancer cells, Warburg attempted to establish the reason for the failure of oxygen to suppress glycolysis. His measurements of the anaerobic and aerobic lactic acid produc tion by various tissues, and the comparison with the measurements of the oxygen consumption, showed that in many tissues approximately two molecules of lactate are prevented from appearing when one molecule of oxygen is taken up by respiration. This is the same quantitative relation which Meyerhof had found in muscle tissue. To express the effectiveness of respira tion in preventing aerobic glycolysis, Warburg introduced the quotient
anaerobic glycolysis minus aerobic glycolysis
respiration
and called it ‘Meyerhof quotient’. When glycolysis is expressed in units of lactate formed and respiration in terms of units 0 2consumed, the value of the Meyerhof quotient is usually two. In the case of cancer tissue he found respiration either less efficient or altogether smaller, and he thus reached the conclusion that in cancer cells respiration is defective in controlling aerobic glycolysis.
At this stage it was entirely obscure by which kind of mechanism oxygen might act in preventing the products of fermentation from appearing and in bringing about a resynthesis of carbohydrate. The time was not ready because the pathways of glycolysis and gluconeogenesis had not yet been clarified. A contribution to the subject of the relations between respiration and fermentation was Warburg’s discovery in 1926 that the link between respiration and fermentation can be severed by a specific inhibitor, ethyl- carbylamine [87]. This substance, he found, does not inhibit at low concentra tion the respiration of many cells, yet at these concentrations cells exposed to ethylcarbylamine glycolyse in oxygen almost at the same rate as in the absence of oxygen. In other words, ethylcarbylamine ‘stimulated’ aerobic glycolysis. On the basis of this finding Warburg visualized a link between respiration and fermentation in terms of a specific chemical reaction and he introduced the term ‘Pasteur reaction’ for this chemical link. He looked upon ethylcarbylamine as a specific inhibitor of the Pasteur reaction. Since ethylcarbylamine readily chelates with heavy metal ions and inhibits other reactions catalysed by heavy metals, Warburg suggested that the catalyst promoting the Pasteur reaction contained a heavy metal. The nature of his hypothetical reaction and its mechanism of action remained obscure for many years in spite of numerous efforts to elucidate it.
Subsequent work initiated by Engelhardt and elaborated by Lynen, Bucher, Lowry, Racker and Sols made it clear that this concept of the mechanism of the Pasteur effect is not correct (28). In fact oxygen does not at all cause a re-synthesis of the products of fermentation. Respiration, through the synthesis of ATP, modifies the catalytic properties of the rate-limiting enzyme of glycolysis, phosphofrucktokinase. The activity of this enzyme is variable and is regulated by the concentrations of ATP, ADP and inorganic phosphate, as well as by various other metabolites. Phosphofructokinase is an ‘allosteric’ enzyme, and the Pasteur effect can now be accounted for by the allosteric properties of this enzyme: ATP inactivates phosphofructo kinase while ADP and inorganic phosphate activate it. Since respiration, through oxidative phosphorylation, causes a conversion of ADP and in organic phosphate to ATP it decreases the activity of phosphofructokinase; further, since ethylcarbylamine, as was eventually established, uncouples oxidative phosphorylation it can abolish the effect of respiration on glycolysis. Many other ‘uncouplers5were found to have the same effect. Thus the ‘Pasteur reaction5 is an aspect of oxidative phosphorylation. Since heavy metals are components of the respiratory chain, W arburg was right in postula ting that heavy metals play a part in the Pasteur reaction.
This answer to the question of the nature of the Pasteur effect was estab lished some 40 years after Warburg had first stated the phenomenon in clear terms; it is now evident that this answer could have been established only after much collateral progress in enzymology had been made.
Warburg’s starting point in studying cancer, it will be recalled, was the question whether respiration rises (as it does in the sea urchin egg) when growth begins. What emerged was no rise in respiration but a different kind of energy supply; a significant amount of energy in neoplastic tissue being derived from glycolysis, even under aerobic conditions. In ascites tumour cells this can be 50% but the totalenergy supply, in terms ofATP production, is no greater in cancer cells than in their non-growing parent cells.
Why, then, is the rate of energy supply unchanged when a normal cell becomes cancerous and begins to grow, whilst there is a great difference between the rates in the non-growing and the growing sea urchin egg ? The reason may be this: the unfertilized non-growing egg has an exceedingly low metabolic rate because cell activities are minimal. On the other hand, the parent cells of cancer cells are usually very active. When they become cancerous the energy supply is diverted from the performance of their normal function to growth. This is an aspect of ‘de-differentiation5, or ‘re orientation of gene expression5. Because of this switch-over from one activity to another no increase in the overall rates of energy supply occurs. In the un fertilized egg there are no significant supplies of ATP which can be re directed. Hence the increase on fertilization.
Warburg continued a keen interest in cancer throughout his life. Following up his earlier work he studied in the 1950s and 60s how the aerobic glycolysis of cancer cells—and he implied cancer itself—may arise. He extended the work of Goldblatt and Cameron (20) who had found that fibroblasts in tissue culture develop into fibrosarcoma cells when repeatedly exposed for short periods to low oxygen pressure. Warburg showed that embryonic mouse cells acquire the metabolic characteristics of cancer cells in tissue culture at low oxygen pressure within 48 hours i.e. in the course of two cell divisions. Most important of all, if the normal oxygen pressure is restored, the cancer metabolism remains and supports growth. Thus the transforma tion of embryonic cell metabolism into cancer metabolism can be irreversible, just as the formation of cancer cells from normal body cells is irreversible (13, 14, 19). On injection into the animal the transformed embryonic cells are well-known to develop into fatal cancers. Such observations led Warburg to the conviction that the loss of normal respiration is a key factor in carcinogenesis.
He was also very much concerned over the indifference of the authorities and the general public towards the prevention of cancer. He pressed on many occasions for measures which make proper use of the scientific in formation. He wrote [477, 494] ‘Many experts will agree that one could prevent about 80% of all cancers if one could keep away the known carcino gens. This might involve very little expense and especially would require little further research’. Already in 1954 he expressed the hope that cigarette smoking, air pollution by motor car exhausts and food additives would be greatly restricted. He was indeed exasperated that so little was being done in this direction and that people continued to argue that too little was known about cancer to take effective preventive action. While no-one will quarrel with these feelings, his views on the significance of the metabolic character istics of cancer cells were not shared by the majority of experts—though none of the facts on which they were based have been refuted.
In 1967 he summarized his views in a privately printed paper ‘The prime cause and prevention of cancer’ [477, 494] which included the following passages:
‘There are primary and secondary causes of diseases. For example, the primary cause of the plague is the plague bacillus, but secondary causes of the plague are filth, rats, and the fleas that transfer the plague bacillus from rats to man. By a prime cause of a disease I mean one that is found in every case of the disease.
‘Cancer, above all other diseases, has countless secondary causes. Almost anything can cause cancer. But, even for cancer, there is only one prime cause. Summarized in a few words, the prime cause of cancer is the replacement of the respiration of oxygen in normal body cells by a fermentation of sugar. All normal body cells meet their energy needs by respiration of oxygen, whereas cancer cells meet their energy needs in great part by fermentation. All normal body cells are thus obligate aerobes, whereas all cancer cells are partial anaerobes. From the standpoint of the physics and chemistry of life this difference between normal and cancer cells is so great that one can scarcely picture a greater difference. Oxygen gas, the donor of energy in plants and animals is dethroned in the cancer cells and replaced by an energy yielding reaction of the lowest living forms, namely, a fermentation of glucose’.
‘The key to the cancer problem is accordingly the energetics of life, which has been the field of work in the Dahlem institute since its initiation by the Rockefeller Foundation. In Dahlem the oxygen transferring and hydrogen transferring enzymes were discovered and chemically isolated. In Dahlem the fermentation of cancer cells was discovered decades ago; but only in recent years has it been demon strated that cancer cells can actually grow in the body almost with only the energy of fermentation. Only today can one submit, with respect to cancer, all the experiments demanded by Pasteur and Koch as proof of the primary cause of a disease. If it is true that the replacement of oxygen-respiration by fermentation is the primary cause of cancer, then all cancer cells without exception must ferment, and no normal growing cell ought to exist that ferments in the body.’
‘Most experts agree that nearly 80% of cancers could be prevented, if all contact with the known exogenous carcinogens could be avoided. But how can the remaining 20%, the endogenous or so-called spontane ous cancers, be prevented?
‘Because no cancer cell exists, the respiration of which is intact, it cannot be disputed that cancer could be prevented if the respiration of the body cells would be kept intact.’
‘Today we know two methods to influence cell respiration. The first is to decrease the oxygen pressure in growing cells. If it is so much decreased that the oxygen transferring enzymes are no longer saturated with oxygen, respiration can decrease irreversibly and normal cells can be transformed into facultative anaerobes.
‘The second method to influence cell respiration vivo is to add the active groups of the respiratory enzymes to the food of man. Lack of these groups impairs cell respiration and abundance of these groups repairs impaired cell respiration—a statement that is proved by the fact that these groups are necessary vitamins for man.
‘To prevent cancer it is therefore proposed first to keep the speed of the blood stream so high that the venous blood still contains sufficient oxygen; second, to keep high the concentration of hemoglobin in the blood: third to add always to the food, even of healthy people, the active groups of the respiratory enzymes: and to increase the doses of these groups, if a pre-cancerous state has already developed. If at the same time exogenous carcinogens are excluded rigorously, then much of the endogenous cancer may be prevented today.
‘These proposals are in no way Utopian. On the contrary, they may be realized by everybody, everywhere, at any hour. Unlike the pre vention of many other diseases the prevention of cancer requires no government help, and not much money.’
These passages on the ‘primary’ cause of cancer, written at the age of 83, still show W arburg’s clear, logical and forceful style but the balance of judgement, in the view of most experts, is at fault. His sweeping generalizations spring from gross simplifications. The partial replacement of respiration by glycolysis is only one of many characteristics which distinguish the cancer cells from normal cells. Warburg neglected the fundamental biochemical aspect of the cancer problem, that of the mechanisms which are responsible for the controlled growth of normal cells and which are lost or disturbed in the cancer cell. No doubt, the differences in the energy metabolism dis covered by Warburg are important, but however important, they are at a level of the biochemical organization of the cell which is not deep enough to touch the heart ofthe cancer problem, the uncontrolled growth. Warburg’s ‘primary cause of cancer’—the replacement of respiration by fermentation—• may be a symptom of the primary cause, but is not the primary cause itself. The primary cause is to be expected at the level of the control of gene ex pression, the minutiae of which are unknown though some of the principles involved are understood.
The catalysts of the respiratory chain
The earlier work on the nature of the catalysts of biological oxidations had convinced Warburg that heavy metals played a key role. He had demonstrated the presence in respiring cells ofiron; he had demonstrated the inhibition of biological oxidations by traces of cyanide which combine with iron compounds and he had established ‘models’ of respiring systems, e.g. blood charcoal, which simulate biological oxidation in that they catalyse the oxidation by molecular oxygen of physiologically occurring stable sub stances and that this oxidation is sensitive to cyanide. In 1926 he added a new piece of evidence in support of this conclusion which subsequently turned out to be decisive. Since some iron compounds, for example haemoglobin, readily react with carbon monoxide he tested the effect of carbon monoxide on the respiration of yeast cells and discovered that this substance indeed inhibits respiration, and that the degree of inhibition depends on the oxygen pressure [115, 128]. Hence at a given carbon monoxide pressure, the inhibition is greater the lower the oxygen pressure, a behaviour exactly analogous to the competition of haemoglobin for oxygen and carbon mon oxide. While these experiments were in progress, A. V. Hill visited the laboratory and drew Warburg’s attention to the light-sensitivity of carbon monoxide haemoglobin discovered by Haldane and Smith in 1896 (21). The test of whether the carbon monoxide compound of the respiratory catalyst was also light sensitive was carried out immediately as the necessary tools were at hand. The result was that the inhibition of respiration was greatly dimin ished when the yeast suspension was illuminated. This phenomenon provided Warburg with the possibility of establishing the spectrum of the catalyst which he exploited with exquisite skill and ingenuity. He illuminated the yeast suspensions with monochromatic light of known intensities and measured quantitatively the effect of light on the inhibition of respiration by carbon monoxide. From these measurements he obtained the absorption spectrum of the catalyst which competes for oxygen and carbon monoxide [140, 148]. The principle involved was simple: since only light absorbed by the catalyst can be effective in removing the inhibition, the absorption spectrum of the catalyst is related to the action of the light. But to devise and to carry out the experiments and to develop the mathematical analysis of the measurements required very exceptional experimental and theoretical skill. First he had to find sources of monochromatic light of sufficient in tensity. Then he needed methods for measuring the gas exchanges and light intensities and finally he had to elaborate the theory for the quantitative interpretation of the measurements. The spectrum found agreed quantita tively with those of iron porphyrins such as haemoglobins and the cyto chromes, but it was not identical with either type. Further, neither the haemoglobins nor the cytochromes are oxidizable by molecular oxygen and in this respect differed from Warburg’s compound, the ‘oxygen transferring enzyme’. It was this work for which Warburg was awarded the Nobel Prize for Medicine and Physiology in 1931.
The discovery of the CO-sensitive iron porphyrin (or ‘haem’), and the effects of light upon this, had to be reconciled with Keilin’s discovery of the three cytochromes a,b and c in 1925, three haem compounds which in cells readily undergo oxidation and reduction, the oxidation being inhibited by CO, and the reduction by narcotics. These cytochromes showed no changes in their absorption spectrum on addition of cyanide or CO, nor was their spectrum identical with Warburg’s haem compounds. This was the origin
of a prolonged controversy between Warburg and Keilin [154,506]. Warburg- argued that the cytochromes are not directly involved in the interaction between O a and the substrates of respiration. The details of this controversy cannot be discussed here and the reader must be referred to the Biographical Memoir of Keilin (33), Keilin’s own record in his book The history of cell respiration and cytochrome (25), and to an obituary notice by E.C. Slater (42).
The apparent contradictions were resolved by Keilin’s discovery in 1939 (26) that cytochrome a consisted of (at least) two components, one of which, named a3 or cytochrome oxidase, combines with CO, HCN and had the same spectrum as Warburg’s ‘respiratory enzyme’ of 1926. Already in 1927,
Keilin had shown that the ‘indophenol oxidase’ (an enzyme of wide occurrence in living material which catalyses the formation of indophenol blue in the presence of a-naphthol and air) is inhibited by CO and by cyanide and that the inhibition by CO is reversed by light. He concluded that indophenol oxidase and Warburg’s enzyme were identical and that this enzyme was responsible for the oxidation of the cytochromes. In 1929 Keilin wrote ‘the term indophenol oxidase must be retained in preference to the term “respiratory enzyme”, which is too wide and which implies that it is the only enzyme taking part in the respiratory mechanism of the cell. This is, however, not the case, indophenol oxidase in the living actively respiring cell represents only one link in the chain forming the complicated respiratory mechanism, in which several other respiratory enzymes and systems are involved and intimately connected’. Warburg resented the suggestion that the iron porphyrin discovered by him should be named after an artificial substrate which it happens to be able to oxidize, when in fact it is a catalyst essential in the vast majority of aerobic organisms for the combustion of organic substances by molecular oxygen.
Warburg’s next major undertaking was the elucidation of the mechanism of action of the ‘hydrogen activating enzyme’ in biological material. It had been known from the work of Thunberg, Wieland and others, beginning in the first decade of this century, that biological material contains enzymes which catalyse the reduction of methylene blue (and similar synthetic dyes) or nitrophenols to colourless compounds by a variety of substances like succin ate, malate, citrate, lactate or glutamate. The common feature of these substances is their ability to donate hydrogen atoms and undergo ‘de hydrogenation’. Such experiments indicated that Oa, the physiological hydrogen acceptor in the process of combustion, can be replaced by other acceptors. During the 1920s it was intensely argued whether the catalytic activation of oxygen, as studied by Warburg, or the catalytic activation of the hydrogen atoms of the substrates, as studied by Thunberg and by Wieland, was the essential feature of biological oxidations. At the earlier stages of the discussion Warburg dismissed the concept of the activation of hydrogen because the experiments of Thunberg and others on which it was based, involved non-biological oxidising agents such as methylene blue or nitro phenols. He scathingly stated that the oxidation of organic material by derivatives of nitric acid or synthetic dyes is of no biological interest. But he did not properly appreciate that although methylene blue is not a naturally occurring compound, the reactions which Thunberg had studied were enzyme-catalysed processes. Eventually, however, Warburg became im pressed by the enzymic nature of the methylene blue reduction when E. S. G. Barron demonstrated some methylene blue experiments on red cells to him personally in 1929 while visiting the Johns Hopkins Medical School at Baltimore. A year earlier Barron and Harrop (4) had observed that ad dition of methylene blue to rabbit erythrocytes promotes an oxidation of glucose by molecular oxygen to C02and pyruvate. In this system methylene blue was reduced enzymically and re-oxidized non-enzymically by Oa. Warburg was intrigued by this phenomenon and after his return to Berlin he began an investigation into the chemistry of the action of methylene blue. Initial experiments showed that Oa was taken up on addition of methylene blue also after cytolysis of the red cells provided that glucose was replaced by glucose-6-phosphate. Thus he obtained the oxidizing process in a homogeneous solution, and this made it possible to investigate the problem by the standard methods of chemistry. Dialysis of the solution indicated that the hydrogen transferring mechanism involved a high molecular com ponent—an enzyme—and a low molecular heat stable component, a co enzyme. Next he discovered that yeast extracts showed a much greater activity under the same conditions and the subsequent work was therefore carried out on this material. The protein component was found to consist of at least two fractions. One of these was yellow and was later referred to as the yellow enzyme’. The second was colourless. On shaking with aqueous methanol the yellow enzyme was denatured and the coloured component went into solution. Warburg crystallized a derivative of the yellow component, luminoflavin which Stern and Holiday (43) subsequently identified as a methylated alloxazine. Luminoflavin arises from the coloured com ponent of the yellow enzyme by illumination in alkaline solution. Ribo-das flavin, a substance which R. Kuhn first isolated from milk in 1933, on illumination forms the same luminoflavin. The yellow enzyme, as Theorell showed in 1934, consists of a specific protein and a phosphorylated ribo flavin, the latter being the prosthetic group which undergoes reversible hydrogenation and dehydrogenation, according to the scheme
where R is ribito]phosphate. The ring system is that of an isoalloxazine. A variety of yellow enzymes, now called flavoproteins, were discovered, all containing riboflavin phosphate, and capable of acting as hydrogen carriers. In some cases the prosthetic group is the same as in Warburg’s first yellow enzyme, namely a riboflavin phosphate or, as Warburg called it ‘alloxazine nucleotide’ of the general structure:
Phosphate—ribitol—dimethylisoalloxazine
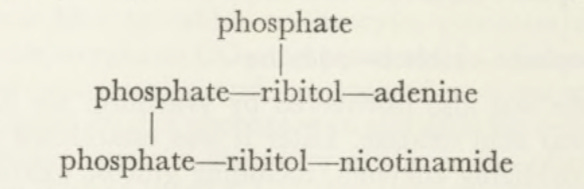
but in the majority of cases the prosthetic group is a ‘flavine adenine dinucleotide’:
Phosphate—ribitol—dimethylisoalloxazine
Phosphate—ribitol—adenine
This dinucleotide was also discovered by Warburg. He identified it when purifying D-amino acid oxidase. Later it was established as the prosthetic group of other oxidizing enzymes, including glucose oxidase, xanthine oxi dase and acyl CoA dehydrogenase. In the latter cases the flavoproteins accept hydrogen atoms directly from organic substrates, but this did not apply to the red cell system where Warburg found the first yellow enzyme. As already mentioned this requires a coenzyme, the nature of which War burg proceeded to investigate, a line of work which led to another out standing achievement.
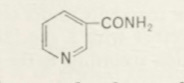
Adapting classical organic chemical methods of isolation Warburg obtained a pure coenzyme in 1934 [225, 235, 243, 255]. It contained nitrogen and phosphorus, and on hydrolysis formed phosphate, pentose, adenine and a substance not hitherto known to occur in coenzyme fractions. This was identified as nicotinamide:
and eventually this turned out to be the catalytically active group of the coenzyme which, by undergoing hydrogenation and dehydrogenation, (oxidized flavin) (reduced flavin) transfers hydrogen atoms. The same substance was subsequently found to be present in the co-enzymes of numerous other oxido-reductions, especially those of fermentations. By now well over 150 different dehydrogenases are known where nicotinamide plays a key role as a hydrogen carrier.
The discovery of nicotinamide and the elucidation of its mode of action was a monumental achievement. Many had tried before Warburg to isolate and identify the active principle in the coenzyme fraction ofoxido-reductions, especially of fermentations. The existence of coenzymes had been known since the pioneer work of Harden in 1906 but its function in yeast fermentation remained entirely obscure. The presence of adenine, pentose and phosphate had already been established by von Euler in impure fractions of the co enzymes of alcoholic fermentations; however as long as the substances were impure, the significance of the components isolated from it remained un certain. Warburg made full use of the facilities for large-scale operations which he had installed in his new institute in 1931. To illustrate the scale of his operation: the starting material for the isolation of the coenzyme was 100 litres of washed horse erythrocytes [243]. These were lysed with 200 litres of water and at once treated with 500 litres of acetone. This yielded 4.8 g of ‘coenzyme’. Micro-tests monitored the coenzyme content at each stage of the purification procedure.
The first pure coenzyme to be isolated by Warburg contained one molecule nicotinamide, one molecule adenylic acid, two molecules of ribose and three molecules of phosphate. It proved to be a dinucleotide of the general structure
Soon afterwards Warburg isolated, also from red cells, a second coenzyme which differed from the first by containing two phosphates only. This was the coenzyme of glycolysis and of alcoholic fermentation. Since the pyridine ring is the characteristic feature of these dinucleotides, Warburg named them pyridine dinucleotides and distinguished between the diphospho- and triphospho-pyridine nucleotides (DPN and TPN). This nomenclature was in use for over thirty years when the International Nomenclature Committee replaced it by the terms nicotinamide adenine dinucleotide and nicotinamide adenine dinucleotide phosphate (NAD and NADP).
The identification of nicotinamide and the coenzyme fraction did not at once explain how the coenzymes act, but the availability of pure substances made it possible to study their reactivity under clear-cut conditions. Glucose- 6- phosphate and TPN, together with a specific protein isolated from red cells led to a prompt interaction according to the equation
pyridine nucleotide + glucose-6-phosphate + H 2 O
-> dihydropropyridine nucleotide + phosphogluconic acid.
Thus hydrogen had been transferred from glucose-6-phosphate to the pyridine nucleotide. The dihydropyridine nucleotide was not autoxidisable but on addition of the yellow enzyme the following reaction occurred
dihydropyridine nucleotide + yellow enzyme
-> pyridine nucleotide + reduced enzyme.
By these two reactions hydrogen is transferred from glucose-6-phosphate to the yellow enzyme, with the pyridine nucleotide acting as a hydrogen carrier. Warburg suspected that this carrier function is brought about by a reversible hydrogenation of one of the double bonds of the pyridine ring although partial reversible hydrogenations of this type were unknown to organic chemists at that time. On the basis of model-experiments, carried out jointly with P. Karrer [251] on N1-substituted nicotinic acid derivatives, the conclusion was reached that the hydrogenation occurs at carbon atom 1 of the ring. Some 18 years later Pullman, San Pietro and Colowick (39), using deuterium, established that in fact carbon atom 4 is the site of hydrogenation.
In the study of the reduction of pyridine nucleotides it was of great importance that on the reduction of the pyridine nucleotide an absorption band of 340 nm arises which provides a convenient spectrophotometric test for the reduction of pyridine nucleotides. The discovery of this absorption band was the basis of a large proportion of the subsequent work on the pyridine nucleotides, especially in the measurements of enzyme activities and the determination of minute quantities of metabolites. Warburg’s collaborators, Negelein and Haas, made use of it when they examined the role of the specific proteins—the dehydrogenases—in the transfer of hydrogen. This work led to the conclusion that the pyridine nucleotides combine with the protein and that the high specificity of the dehydrogenases resides in the protein part. Today this is taken for granted, but this was not so in the early 1930s, as Willstatter’s statement of 1926 (48) illustrates ‘Enzymes are neither proteins, nor carbohydrates, nor do they belong to any of the known large groups of complex organic compounds’.
Thus Warburg’s discoveries of the yellow enzyme and the pyridine nucleotides solved key aspects of the mechanism of action of dehydrogenases.
Crystallization of enzymes of fermentation
By the early 1930s, thanks to the work of Harden, Neuberg, Meyerhof, Embden, the Coris, Parnas, Needham and Lohmann, the enzymes of the intermediary stages of lactic and alcoholic fermentations had been identified and their reactions had been formulated, but not a single one of the enzymes had been obtained in a pure crystallized form. Since the ultimate analysis of the nature of enzyme action depends on the availability of pure substances, the purification of enzymes is of crucial importance. Only with pure sub stances can the stoichiometric interactions between enzymes, substrate and coenzymes be analysed.
Warburg, together with his collaborators, Christian, Kubowitz, Negelein and Bucher, tackled enzyme purification with new procedures and was the first to succeed in crystallizing enzymes connected with fermentations— no less than 9. In the now accepted nomenclature they were lactic de hydrogenase, enolase, aldolase (from muscle and from yeast), glyceralde- hydephosphate dehydrogenase, 3-phosphoglycerate kinase, alcohol de hydrogenase, pyruvate kinase, a-glycerophosphate dehydrogenase, and triose phosphate isomerase. Others, e.g. glucose-6-phosphate dehydro genase, were highly purified.
The starting points for this striking progress were the new tests of enzyme activity. Prior to Warburg, the quantitative assay of enzyme activity, essential in the process of purification because it indicates the enzyme content of each fraction, was usually very laborious. It involved the chemical determination of substrate or product changes which often required hours rather than minutes and consumed considerable amounts of valuable material. The new optical tests could be carried out in minutes on micromole quantities. Since in the key steps offermentation reduced pyridine nucleotide is either formed or removed, these steps can be tested quantitatively by measuring changes in the absorption at 340 nm. Furthermore steps not involving pyridine nucleotides can be coupled to such a reaction and thereby become amenable to the optical test. A second absorption band which Warburg found useful is the enol band of phosphopyruvate at 240 nm. He introduced many other methodological details such as the use of protamine for the removal of nucleic acid from protein solutions, a technique now commonly applied.
A consequence of the work on enzyme isolation was the discovery of a new key intermediate offermentations: 1,3-diphosphoglycerate, a discovery only possible after the purification of the glyceraldehydephosphate dehydro genase [288]. The purification of the enzymes also led to the clarification of the inhibition of fermentation by fluoride. Lohmann and Meyerhof had established that fluoride specifically inhibits enolase. Using the pure enzyme Warburg showed that the inhibitory substance is a Mg-fluorophosphate which combines with the enzymes [293].
The crystallization of the enzymes of fermentation and of the coenzymes not only elucidated finer details of the intermediary steps of fermentations but also had far-reaching practical consequences. It provided powerful microanalytical tools, the crystalline enzymes being extraordinarily specific and highly sensitive agents for the analysis of cell constituents. Such reagents proved decisive in many fields of research, as well as in clinical biochemistry.
Photosynthesis
Warburg’s general interest in biological energy transformations led him in 1914 to start a study of photosynthesis—the most important of all energy transforming mechanisms in living matter inasmuch as all life depends on it.
It was one particular aspect of this field which fascinated him: the thermo dynamic efficiency ofphotosynthesis. Connected with this was the problem ofthe nature of the process in which light participates, the ‘photochemical reaction’.
In 1912 Einstein had formulated the law of the photochemical equivalence according to which a photochemical reaction involves primarily the absorption of 1 quantum per ‘photolyte’, i.e. the substance becoming reactive after absorption of light. Soon afterwards, Emil Warburg provided the first experimental evidence of the validity of this law in studies on the photo chemical fission of HBr and HI. Otto’s close personal contacts with these developments provided the basis of his approach to the study of photo synthesis. It was evident to him that this problem could only be tackled by new methods of measurement. The necessary physical methods for quantitative determinations of light intensities—bolometry—had been worked out in his father’s laboratory where he learned these techniques. Light intensity measurements had to be correlated to measurements of the rate of photo synthesis and when Warburg entered the field reliable quantitative methods for measuring the rates under a variety of conditions were hardly available.
He introduced several decisive innovations. First, he modified the mano-metric technique (which he had already used for the measurement of cell respiration) to the study of photosynthesis. Secondly, he adopted suspensions of a uni cellular a l g a — l lalrehoC— as experimental material, having in earlier work found suspensions of other living cells—erythrocytes, yeast, staphylococci—a very valuable material for quantitative work. Before him, investigators had used leaves which do not lend themselves readily to quantitative experiments. Suspensions of lreaohC,which can be grown under controlled conditions and can be handled like solutions, were an ideal material for quantitative work on photosynthesis, and have ever since been a favoured object. Thirdly, he developed the method of intermittent illumination which proved essential in establishing conditions where the ‘light reaction’ (or reactions) rather than the ‘dark reactions’ of photosynthesis are rate-limiting. Fourthly, he introduced bicarbonate-carbonate buffers which solved the problem of controlling the CO2 supply at low CO2 concentrations. Fifthly, he developed the use of inhibitors and discovered that the ‘photochemical reaction’ is especially sensitive to narcotics while the dark reactions, like respiration, are sensitive to cyanide.
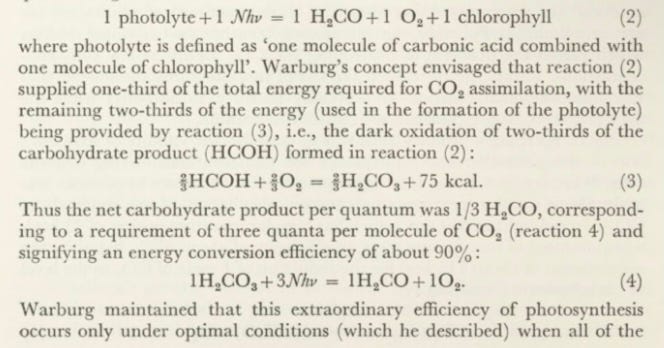
One of Warburg’s primary aims was to establish the validity of Einstein’s law of the photochemical equivalence for photosynthesis. Using Chlorella cells, Warburg found that four light quanta were necessary to produce one molecule of oxygen (corresponding to the assimilation of one molecule of C 0 2). In red light at 660 nm, which has a molar quantum energy of 43 kcal, a requirement of four quanta gave an efficiency of about 65%, based on the requirement of about 115 kcal for the reduction of 1 mole of C 0 2to the level of carbohydrate (equation 1):
The finding that about 65% of the absorbed radiant energy can be transformed into chemical energy was remarkable not only for its high efficiency but also for the requirement of four collaborative quantum absorption acts to bring about the evolution of one molecule of 0 2—a situation in conflict with Einstein’s law which postulates that the primary process in any photo chemical reaction involves the absorption of a single quantum. It was on these grounds that already in 1926 Henri (22) suggested that oxygen evolution by whole cells (i.e., complete photosynthesis) is unsuited for measurements of the quantum efficiency of the primary photochemical act in photosynthesis. Warburg responded by postulating that C O 2 molecules remain adsorbed on the chloroplast surface until successive stepwise reductions by light-activated chlorophyll convert them to glucose and liberate oxygen. By invoking such a mechanism, Warburg continued to regard the oxygen evolution that accompanies C O 2 assimilation as a valid measurement of primary photo chemical reactions.
In later years many investigators who followed Warburg’s methods ob tained much lower values for the quantum requirements of photosynthesis: ten or more quanta per molecule of oxygen evolved. These results did not shake Warburg’s conviction about the unique efficiency of photosynthesis. During the last 20 years he vigorously reaffirmed his earlier findings and, together with D. Burk [325, 326, 329], advanced them to the point of dividing the photosynthetic energy conversion process into two parts: (a) a one- quantum light reaction which liberates oxygen and converts a bound species of C 0 2 into carbohydrate; and (b) dark oxidative reactions which provide the rest of the total energy needed for COa assimilation. He viewed the one- quantum light reaction as the long-sought primary reaction that conforms to Einstein’s law of photochemical equivalence. In his last major paper on photosynthesis, published in 1969 [497], Warburg represented this one- quantum light reaction as:
chlorophyll is present as the photolyte, i.e., the chlorophyll-carbonic acid complex. Lower efficiencies of photosynthesis, he reasoned, result from light absorption by a portion of chlorophyll that has not been converted to photolyte and is therefore photochemically inactive.
However, these concepts of the bioenergetics of photosynthesis have not gained general acceptance. Biochemists could not accept a mechanism for a one-step transformation of a compound at the level of carbonic acid to carbohydrate. Moreover, more recent research has amassed convincing evidence in favour of the reductive pentose phosphate cycle as the pathway of photosynthetic carbohydrate synthesis from C 0 2. Furthermore, evidence has been accumulated in recent years that one-quantum light reactions in photo synthesis are concerned not with the terminal events of oxygen evolution and
C 0 2 assimilation but rather with intermediate light-induced electron transfer steps (2, 3, 24).
The great majority of investigators agree with van Niel (36) that the photochemical reaction is a fission of water and not a direct reduction of C 0 2. Water is taken to be split according to the reaction
where A is a hydrogen acceptor. Direct experimental support for this concept came from experiments by R. Hill in 1939, who showed that isolated chloroplasts on illumination can form 0 2provided that a hydrogen or elec tron acceptor such as Fe3+ e.g. ferric oxalate, is present. In 1944, Warburg and Liittgens [315] found that quinone is a more effective hydrogen acceptor than Fe3+. In 1960 Warburg showed that the Hill reactions are accelerated by catalytic amounts of C 0 2 [427], and on the basis of this finding, Warburg remained convinced that the light reaction is a photolysis of C 0 2 according to reactions (1) and (2) and he chose to ignore the powerful argument that photosynthetic bacteria, which can also convert C 0 2 to carbohydrate in the light, do not produce 0 2. Of course they require an electron acceptor in place of 0 2, but sulphur compounds and H+ can function as such. The
primary hydrogen acceptor in both cases is probably ferredoxin.
During the last years of his life Warburg talked several times about ways and means of bridging the gaps between his own views and those of others— without publishing the outcome. He visualized a conciliation of the divergent views on the basis of the fact that the Hill reaction depends on catalytic amounts of COa. This, he thought, may suggest that C 0 2participates in the Hill reaction as a catalyst, and as the Hill reaction is an oxido-reduction, the role of COa may be that of an electron carrier. He had already formulated such a role in 1968:
H2C03+light ^HCOH +O, 2NADP+HGOH+2H20 ->2NADPH2+H2C03
sum: 2NADP + 2H20 + light -» 0 2+ 2NADPH2.
According to this mechanism carbonic acid would undergo reversible reduction and oxidation, and be regenerated after each catalytic cycle; it would not be converted to carbohydrate. This leaves room for the Calvin cycle (or analogous sequences).
Thus some aspects of the controversies may well resolve themselves on the grounds of the well-established fact that CO2 is involved in photosynthesis in (at least) two entirely different ways, in a photochemical reaction (where it is a catalyst and may well react in the form of Warburg’s postulated photolyte)
and in the dark reactions of the Calvin cycle.
Meanwhile, Warburg’s views on two interlinked aspects of photosynthesis
—the energetic efficiency and the nature of the photochemical reaction— remain controversial. Nevertheless, despite what many would consider aberrations ofjudgment there is wide agreement among his critics that War burg’s pioneering contributions to the experimental progress of the subject have been monumental.
Warburg’s contributions to photosynthesis were not limited to the ener getics of the process. Working with isolated chloroplasts he discovered in 1946 that chloride is an essential cofactor in the Hill reactions [315]. At that time it was not yet known that chloride is essential for plant growth. This is perhaps the only case in the field of plant nutrition where the site of action of a plant nutrient was established before it was known to be an essential nutrient. The essentiality of chloride for plant growth was established several years later (7, 34). The precise role of chloride in the Hill reactions remains one of the unanswered questions in photosynthesis.
Nitrate reduction green plants
In addition to his work on photosynthesis Warburg made another im portant contribution to plant biochemistry through his studies of the mechanisms of the reduction of nitrate to ammonia [45]. Nitrate is reduced readily in the dark by plants and by non-photosynthetic organisms, e.g. bacteria, but light was known to accelerate the process in plants. Before Warburg, quantitative information on the subject was limited because it was not possible to measure the rate of reduction accurately under controlled conditions, mainly because oxidising processes very much predominated in cells which were capable of reducing nitrate. Warburg devised a simple technique which increased the rate ofnitrate reduction to such an extent that it became the predominant process. He suspended Chlorella in mixtures of nitrate and nitric acid (10:1). On balance the following reaction took place
HN03+HaO ->NH3+202. (5)
The evolution of oxygen or the formation of ammonia can be easily measured. Whilst the reaction occurs in the dark it is greatly accelerated by light. Warburg’s investigations showed that the mechanism of this process is not
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
Otto Heinrich Warburg 657
adequately expressed by the balance equation. Nitrate reduction is inter linked with the metabolism of carbon and, on illumination, with photo synthesis. The first step is a dark reaction in which nitric acid interacts with organic substances to form ammonia and C 0 2:
HN03+H20 +2C ->NH3+2C02. (6)
Another step is photosynthesis which regenerates organic carbon from C 0 2:
light
2C02 -
> 2C+02. (7)
The balance of these two reactions is identical with the balance of nitrate assimilation (reaction 5). Since the carbon used in reaction (6) is regenerated in the light, small amounts of carbon can reduce unlimited amounts of nitrate in the light. This work gave an answer to the mechanism by which light can accelerate the reduction of nitrate. It laid the foundation for subsequent work in other laboratories which established the key role in nitrate reduction of electron carriers such as NADPH.,, flavoproteins and ferredoxin (see (6)).
Ferredoxin
Warburg was also involved in the discovery of the electron carrier ferre doxin mentioned in the preceding section. This substance is distinguished by its strongly negative potential, close to that of hydrogen gas ( =
—0.420 V at pH 7.0). Its discovery has several roots. The first experiments
dealing with this substance were carried out in Warburg’s laboratory by
Kempner & Kubowitz in 1933 who discovered that the butyric acid
f e r m e n t a t i o n i n Clostridium butyricumis r e v e r s i b l y i n h i monoxide and that this inhibition is reversed by light [217]. This light effect
made it possible to measure the ‘action spectrum’ of the catalyst: it was not that of an iron porphyrin but that of a non-haem iron protein. In dependent of this work was the discovery in 1952 by Davenport, Hill and Whatley (12) of a factor which eventually proved to be ferredoxin catalysing the reduction of methaemoglobin by chloroplasts. Again independent was the work by Mortenson, Valentine & Carnahan (35) in 1962 leading to the isolation of an iron-containing enzyme from Clostridium. This contained 2.5% iron and was named ferredoxin. At the same time Gewitz and Volker [453] in W arburg’s laboratory isolated a ‘red enzyme’ from Chlorella which contained 1% iron and produced one molecule of H 2S per atom of iron on addition of dilute sulphuric acid. Such a release of H2S was later found to be the property of ferredoxins from all other sources. Gewitz and Volker also established an inhibition of photosynthesis by carbon monoxide and noted that the ‘action spectrum’ of the inhibited catalyst was that of a non-haem iron protein very similar to that found by Kempner & Kubowitz in Clostridium butyricum.
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
658 Biographical Memoirs Clinical biochemistry
In 1927 Warburg [108] designed a method ofdetermining trace quantities (10_4mg) of Fe and Cu based on the catalytic effects of these ions on the oxidation of cysteine by molecular oxygen, and with these methods he established the basic facts concerning the occurrence in biological material of free or ‘loosely bound’ iron and copper (i.e. Fe and Cu which readily chelate with cysteine). He discovered that Fe and Cu are regularly present
in human and animal blood plasma and that their concentrations under normal conditions are as constant as are other blood constituents. He estab lished the normal range which for both Cu and Fe is of the order of 1 mg/ litre and found that the concentration of copper is raised in pregnancy, infections and iron deficiency anaemias and that the Fe content falls in iron deficiency anaemias [116, 118]. This work was the starting point for the clinical application of Fe and Cu determination in human serum or plasma, a matter of diagnostic importance especially in Wilson’s disease where the Cu content is greatly decreased. Warburg was the first to detect enzymes in blood plasma which normally function only within living cells. These were, in the first instance, lactate dehydrogenase and aldolase. As they have no function to perform in the plasma. Warburg reasoned, they must be derived by a leakage from cells, or by a disintegration of cells. The detection of the enzymes was possible because of the highly sensitive optical tests. Warburg also found in 1943 that the aldolase content of plasma can rise in pathological states e.g. in tumour-bearing rats. Years later, enzyme assays in blood plasma became established methods of clinical biochemistry. In his book Enzymes in blood plasma, B. Hess (23), wrote in 1963: ‘A decisive stimulus to clinical enzymology was given by Otto War burg in 1935 as a result of the discovery of optical means in the determina tion of enzyme activity, and again in 1943 by the demonstration of enzymes of glycolysis in the serum of normal and tumour-bearing rats. As early as 1943, Warburg and Christian opened up the entire biochemical and bio logical problem of the phenomenon of serum enzymes’.
Other practical applications
Some of Warburg’s work, although aimed solely at gaining knowledge of the laws of nature, had other far-reaching practical and economic con sequences. His discovery of nicotinamide as a cell constituent in 1935 led two years later, in the hands of Elvehjem, to its identification as the anti-pellagra vitamin in liver extracts and to the possibility of preventing and curing pellagra. In 1945 Chorine, when studying the effects of vitamins on myco bacteria discovered the powerful inhibitory effects of nicotinamide on the mycobacteria responsible for leprosy and tuberculosis (9). This was the starting point for systematic investigations into the antitubercular effects of
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
Otto Heinrich Warburg 659
substances related to nicotinamide and in the laboratories of the Hoffmann- LaRoche Company led to the discovery that isonicotinic acid hydrazide (‘isoniazid’) is even more effective and has less side effects than nicotinamide. This drug is now widely used in the control of tuberculosis.
Warburg’s research on the crystallization of enzymes and on measuring their activity by spectrophotometric methods was the basis of a major industrial development—the manufacture and marketing of pure enzymes and of their cofactors and substrates. Crude enzyme preparations, such as urease, pepsin, and pancreatin had been marketed for many decades, some for medical purposes, others as useful chemical agents, but their scope was limited. The manufacture of crystalline enzymes and the development of the spectrophotometric methods provided much more specific—in fact, uniquely specific—and much more sensitive tools. The replacement by fluorometry of spectrophotometry further increased sensitivity by orders of magnitude.
Dr H. U. Bergmeyer who, beginning in the late 1940s, built up the enzyme manufacture of the Boehringer Mannheim Company states that without Warburg the enzyme branch of Boehringer Mannheim would not exist. ‘We consider ourselves as the executors of Warburg’s pioneer work’. A little earlier, in 1947, Dr Charles C. Worthington had started to initiate other aspects of industrial enzyme production in the United States. He had been trained in the laboratory of Dr A. Kunitz at the Rockefeller Institute in Princeton where hydrolytic enzymes were first crystallized on a major scale, and began with the manufacture of trypsin, chymotrypsin, ribo- nuclease and deoxyribonuclease. In 1954 the Seravac Company of South Africa began to manufacture some of the enzymes which Warburg had first purified and crystallized (alcohol dehydrogenase, lactate dehydrogenase, malate dehydrogenase). A Managing Director of the Company, Dr B. R. Cookson, testifies that Warburg’swork was vital to the enterprises.
Warburg himself of course never set out to create a new branch of the chemical manufacturing industry. He needed the pure enzymes in order to study fundamental processes in living cells. He did not even think of suggest ing to firms that they might manufacture enzymes. This was done by Bucher, who approached the Boehringer-Mannheim Company. This account illustrates the frequent experience that first rate fundamental research, sooner or later, leads to some important practical applications.
The last decade
Right to the end of his life Warburg published experimental papers at regular intervals at the rate of about five per year, in the later years mainly on photosynthesis and on cancer. In recognition of his outstanding achieve ments the Max Planck Gesellschaft had waived the usual retirement regulations in his case and fixed no age limit. Although he spoke occasionally of retirement it was his view that as long as he could personally control the work in the laboratory he should carry on, but he announced that he would
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
660 Biographical Memoirs
retire as soon as he felt that he could no longer check every detail personally. He never felt that that time had come. Before retiring he hoped, so he stated, to complete the work on the quantum physics of photosynthesis and to find the cure for cancer. This, he thought, should be possible.
As his bibliography shows the papers of the last period still contain many valuable contributions to both fields of his choice. An example is the discovery of the involvement of COa in the Hill reaction [427], made in 1960 when he was 77. But most of the work was no longer of the pioneering quality of his first 45 years of research. While pursuing ideas based on the old concepts he lost contact with the main stream of progress. In both fields new aspects came to the forefront. Warburg’s concern was almost exclusively with the energy aspects which at the time when he began were indeed outstanding problems, and he limited his interests to the overall balance of energy changes. He took no serious interest in the intermediary steps of the energy transforma tions in photosynthesis, or the general biochemistry of growth and the mechanisms which control growth.
While his drive, industry and experimental skill remained, his ability to keep abreast with the wider aspects of his fields and to judge objectively the merits of his ideas and experimental observations towards the end lost the former high standards. If he lost contact with the newer developments it was perhaps in part due to his unwillingness to surround himself with independ ent fellow scientists and to listen to their points of view, their criticisms, their approaches and their interests. His technical collaborators were a tremendous support to him but they could not act freely in a critical and independent role. So he forewent the benefits which all scientists derive from free and frequent contacts with their equals and their students, including the youngest among them.
Warburg was occupied with his work almost until the last day of his life. On 24 July, 1970, he decided to stay at home because he did not feel well and on the next day he felt a pain in the leg, which had suffered a fracture of the neck of the femur in November 1968. The doctor diagnosed a deep vein thrombosis. Warburg stayed at home and continued to read and to write until 1 August when he felt rather weak. Late in the evening of that day a pulmonary embolus suddenly ended his life.
Personality
General characteristics
Warburg’sgreat achievements in research, extending over a period ofsome 60 years, are the outcome of a combination of an exceptional intellect with a powerful determination to devote his life to experimental science. Ingredi ents of his intellect were a penetrating intelligence, an original, imaginative approach to any situation and independence of commonly held beliefs,
judgements and prejudices. Like Pasteur, whom he admired greatly, he was
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
OttoHeinrich Warburg 661
impelled by a powerful urge. His involvement with science was the dominant emotion of his adult life, subjugating virtually all other emotions. He never married. As a young student in Freiburg he was for some time deeply in love with the daughter of family friends, but he decided, as he did later on a second occasion, that marriage was incompatible with his professional ambitions.
Warburg’s habits were very regular. He paid much attention to his health and fitness. For many years he would get up at 5.30 a.m. From 7.00 to 8.00 (when there was daylight) he would ride on horseback and by 8.00 he was in the laboratory. Except for a lunch break he remained at the bench until late in the afternoon, 6.00 p.m. or so, and woe betide fellow workers who left the laboratory earlier. He had a two month holiday period every year in August and September when he went (until 1944) to his country house on the island of Riigen. There he walked, rode, and spent much time on reading and writing his papers. He took all his notebooks with him. In later years, after the partition of Germany, he went instead to a country estate belonging to Jacob Heiss in the Hunsriick Hills, south of the Moselle valley.
Horse riding was his main physical recreation. He kept his own horses, often three, and even during World War II he managed to keep two, with the help of the officers of a Cavalry Regiment. In 1924 he fractured his pelvis when falling from his horse and for some time lay helplessly in the woods; since then he never rode without the company ofJacob Heiss. When in 1955 Heiss had to give up riding after an operation, Warburg built a riding arena in his own garden, and from this time onwards Warburg only kept one horse, a Hanoverian grey mare. He regularly rode until he was 85, when in an accident in his library he fractured his femur in falling from a ladder.
In 1952 Warburg started a new sport, sailing almost every Sunday on the River Havel and this he continued until he fractured his femur in 1968. He was quite fanatical about this activity and intended to take it up again but did not sufficiently recover from his accident, and he parted with his boat only in May 1970. Fie was also fond of walking in Berlin’s surroundings, especially on winter Sundays, and he was very much attached to the land scape around Berlin with its many lakes and woods. By special and most exceptional courtesy of the Government of the German Democratic Republic
(mediated by Professor Manfred von Ardenne), he had a pass permitting him to roam at any time outside the city of Berlin, a measure of the respect in which he was held.
He was an eccentric in his personal style, aristocratic in outlook in the Greek sense of aristocracy, i.e. rule by the best, and in consequence he was masterful in his bearing. His mind was broadly based on a wide knowledge of history and literature. He was particularly fond of biographies. Much of his reading was in English.
After 4 years of war service in a crack cavalry regiment, he was much attracted by what is best in the military mind: by discipline in manners, by straightforwardness in thought and argument, by the elimination of humbug
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
662 Biographcial Memoirs
and inefficiency. A military leader, he said, is relieved of his office if incom petent. In academic life, many senior people get away with a lot of in competence—technical and moral—whereas an Army officer would not. A soldier of whatever rank is at his post: his academic colleagues, Warburg noted, take ever so many liberties in absenting themselves from their primary duties. So he had rather more friends among soldiers than among university professors. But none of his friendships seemed a close human relationship, except that with Jacob Heiss, his devoted companion from 1919 to the end. In contrast he had great affection for his horses and dogs. Norman, an exceptionally large black and white Great Dane weighing 90 kg—the last of a series of Great Danes—slept on a couch in his bedroom and was given a piece of the best chocolate for his nightcap.
He was essentially a solitary man, self-sufficient, self-contained, never bored with his own company. His work, sports, reading, listening to his record collection, especially Beethoven and Chopin, occupied him to the full. Altogether he was a happy man right to his end. He found the greatest satisfaction in his successful creative work.
Warburg was most intent upon centering his life on matters which seemed to him worthwhile. He intensely disliked wasting time and carefully guarded himself against time-wasting activities and intruders who might waste his time. This showed itself in the manner in which he kept time- wasting intruders away. To an unsuspecting reporter who came to the laboratory asking for an interview he said ‘Professor Warburg cannot be interviewed: he is dead. Good day’. He disposed of a loquacious colleague who confided ‘I wish to lay all my cards on the table’ by saying ‘Sorry, I never play cards. Good day’.
Throughout his life he typed his papers and letters himself, and each paper was typed many times. The Institute employed secretarial help on a part-time basis only; this was for business aspects and not for typing papers. He did not regard his typing as a waste of time.
His stature was short, but his bearing was upright. His speech was clear and to the point, without frills. There was a striking contrast between the fierce, aggressive and self-righteous fighter whom his fellow scientists know from the literature and conferences, and the Warburg in private social life or, as already mentioned, in the relaxed atmosphere of his laboratory. Socially Warburg had an exceptional charm when the company suited him. His conversation which could cover a wide range was direct, original, pene trating and often had a humorous and highly amusing flavour. The direct look from his bright blue eyes was both engaging and fascinating. Though very much of a bachelor he could be particularly gentle, considerate and understanding with women.
People found it puzzling that Warburg in spite of his Jewish ancestry remained unharmed by the Nazis as far as his personal safety and his research work were concerned. One reason is that his mother’s family was non-Jewish; another that Reichs-Marshall Goring (in accordance with his principle ‘I
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
Otto Heinrich Warburg 663
decide who is a Jew’) arranged for a re-calculation of Warburg’s ancestry, and ruled that he was a quarter-Jew. Perhaps the hope of a cancer cure from Warburg influenced this ruling. As a quarter-Jew, Warburg was not permitted to teach in a University, or to hold responsible administrative posts (which suited him very well), but he was not prevented from working in the seclusion in his laboratory. During the war years another high Nazi official—Reichsleiter Bouhler, at one time chief of Hitler’s chancellery— protected Warburg on several occasions when he had been denounced for having spoken critically of the regime.
Outlook on research and on scientific writing
Defining his outlook in research he wrote ‘a scientist must have the courage to attack the great unsolved problems of his time. Solutions usually have to be forced by carrying out innumerable experiments without much critical hesitation’ [467]. This attitude was based on a supreme mastery of experi mental resources and technical skill: it enabled him to devise new experi mental tools and thus to approach the problems in a novel manner. He could penetrate into areas which had not been explored before.
Warburg’s scientific prose was highly characteristic. It was distinguished by an exceptional clarity, achieved by simplicity, brevity and rigid scientific logic. Occasionally striking and colourful passages enlivened the text. Thus, when he discussed the measurement of the ‘action spectrum’ of the carbon monoxide compound of the oxygen transferring enzyme within living cells he wrote ‘if one uses reagents which react only with the enzyme and not with any other cell constituents then these cell constituents do not interfere any more than the wall of a test tube interferes with a chemical reaction. Hence enzymes can be studied like pure substances and from their reactions conclusions about their compositions can be made’.
His aim was to formulate conclusions reached from his experiments without too many qualifications, and he pursued his experiments until this was possible. Too many qualifications, he felt, detract from the value of any statement. In his eagerness to be clear and straightforward he occasionally tended to over-simplify for which he was criticized. This was somewhat deliberate because he subscribed to the view of Bayliss (5):
‘Truth is more likely to come out of error if it is clear and definite, than out of confusion, and my experience teaches me that it is better to hold a well understood and intelligible opinion, even if it should turn out to be wrong, than to be content with a muddle-headed mixture of conflicting views, sometimes called impartiality, and often no better than no opinion at all’.
Scientific controversies
Within his laboratory, in the day to day contacts with his collaborators, Warburg was always friendly and helpful, as long as he was treated with due respect which came naturally to his contacts. He was open, and freely
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
664 Biographical Memoirs
discussed science, public affairs, literature and people. Outstanding was his generosity to his collaborators. Many pieces ofresearch to which he made the main contribution and which he had written, were published without his name, except perhaps in an acknowledgment by the author. A review of his work which established the oxygen transferring catalyst of cell respiration as an iron porphyrin ended with the passage: ‘In concluding I wish to emphas ize that the results which I have presented are largely due to the work of my collaborators, Drs Negelein and Krebs’ [144]. Yet the whole work was conceived by Warburg himselfand the greater part ofthe critical experiments was carried out by him with his own hands.
But when it came to scientific arguments Warburg was a very forthright fighter for what he regarded as the truth. It was his aim to live up to the standards set by his teachers, Emil Fischer, Nernst, his father and van’t Hoff—to be purely objective, personally detached and rigidly truthful. ‘Sachlichkeit und Wahrheit’ were his ideals. He said that his teachers would throw out any collaborators who had not come up to the proper standards of reliability and good behaviour. In his controversies he crossed swords with many of the leading authorities of his time—Willstatter, Wieland, Keilin, von Euler and Weinhouse among them. His style in controversy was often scathing, sarcastic and deliberately ridiculing. He believed that it was dangerous not to contradict erroneous criticisms. He quotes in this context McMunn who in 1885 had discovered in animal tissues what is now called cytochrome [506, p. 61]. McMunn was severely criticized by Hoppe-Seyler
(a leading authority in the field) who argued that haemoglobin is the only red substance in animal tissues and that the ‘histohaematin’ofMcMunn was an artefact derived from haemoglobin. McMunn remained silent and, as a result of this, progress was delayed for over 30 years, until Keilin, in dependently, rediscovered the cytochrome.
To give examples of Warburg’s controversial style: On the question of whether peroxidase (now known to be an iron porphyrin) contains iron, Warburg had stated that some observations, especially the inhibition of the enzyme by cyanide, suggested the presence of iron. This was disputed by Willstatter who found that there was no strict parallelism between enzyme activity and iron content during the early stages of purification. Warburg commented ‘With equal justification Willstatter might have concluded from the C- or N-content of his preparations (which also failed to go parallel with the activity) that the enzyme contained no carbon or nitrogen ’ [506, p.54].
In an obituary ofJames Franck, the distinguished physicist, Kroebel (29), wrote ‘Unfortunately, Franck’s work on photosynthesis was clouded by a quarrel with Otto Warburg which was unpleasant in form. It concerned Warburg’smeasurements ofthe quantum yields which according to Franck’s theoretical physical considerations could not be correct, so that it still remains to be clarified what precisely was measured in Warburg’s experiments’. Warburg’s reply contained the following passages [466] :
‘What “was measured” was the light energy absorbed by the green algae
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
Otto Heinrich Warburg 665
and the oxygen released because these two values are necessary for the calculation of the quantum yield. By contrast nothing “was measured” by Franck, neither the light energy absorbed nor the oxygen released. Franck merely calculated the quantum requirement of photosynthesis on the basis of his theories. Thus Franck attempted to guess how many quanta are neces sary to release one molecule of oxygen. His result was that 8 quanta are required, and when our measurements showed that one quantum is needed the theories of Franck had to be abandoned.
‘Kroebel was correct in writing that our measurements clouded the work of Franck but he is not right in calling our measurements “ unpleasant quarrel” . What can be more pleasant in science than experiments which dispose of wrong theories?’ However, the great majority of experts in the field would not accept this reply as a final comment and would agree with Kroebel that what Warburg actually measured required clarification.
In 1956 Sidney Weinhouse (47) discussed the significance of the high aerobic and anaerobic formation of lactic acid by cancer cells and the failure of respiration to prevent aerobic glycolysis. Warburg’s reply [379] contains the passage ‘Weinhouse is not appreciative of the many important discoveries made since 1925. Most of his comments might have been written before 1925 . . . Obviously nothing could be less enlightened than the opinion of Weinhouse that the respiration of cancer cells is high’. In fairness I should emphasize that Weinhouse’s assessment is accepted by most experts.
W arburg paid little attention to the suggestion that this kind of controversy was unfruitful. Rationally he was fully aware of the futility of emotional controversy. More than once he quoted Max Planck’s dictum (38). ‘A new scientific truth does not triumph by convincing its opponents and making them see the light, but rather because its opponents eventually die and a new generation grows up that is familiar with it’. And as the motto of his book Heavy metals Warburg [506] chose a passage from a remark of Pasteur referring to Bertholet:
‘Comment n’a-t-il pas senti que le temps est le juge souverain? Comment n’a-t-il pas reconnu que du verdict du tempsje n’ai pas a me plaindre?’This referred to a controversy over the nature of alcoholic fermentation— whether living organisms are necessarily involved as Pasteur had claimed or whether fermentation is brought about by soluble enzymes as Bertholet and some others had argued.
Warburg’s involvement in polemics did not spring from doubt over the validity and reproducibility of his experimental findings. It arose over the interpretation of his results, in particular of their full significance. He provoked such criticism because he tended to claim that his were the definite solutions to the problems in hand, when in fact it may have been a partial solution only.
Also characteristic of his feelings towards fellow scientists was his reaction when he had the unusual experience of reading his own obituary in the Times of 12 January 1938. An error had occurred: he had been mistaken for a
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
666 Biographical Memoirs
distant relation, the distinguished botanist and Zionist leader, also called Otto Warburg (1859-1938). The error was corrected on the following day with appropriate apologies. Warburg was mildly amused and mildly annoyed when, as a subscriber to the iseTm,he read the news of hi because he thought that deliberately no reference had been made to what
he considered to be one of his most important discoveries, that of nicotin amide in the co-enzymes of dehydrogenases and the chemical nature of its reversible reduction and oxidation. He commented ‘it looks as if the obituary was written jointly by poor old Heinrich Wieland and poor old Torsten Thunberg’. These two scientists had published much work on dehydrogena tions without establishing the chemical mechanism and, quite wrongly, Warburg considered them his adversaries.
One doubts whether he would have derived more satisfaction from the scanty and largely irrelevant obituary in the Times of 4 August 1970.
Warburg’s litigiousness and concern about being right also expressed itself sometimes in quite gratuitous and pompous pronouncements interspersed in his experimental papers, such as ‘we do not hesitate to express our satisfaction that truth once again has won a war. This time it was a 46 years’ war on bioenergetics’ [497]. His outlook on controversies in science is well expressed by remarks he made in the recorded interview of 1966: ‘Since every real discovery in science means a revolution where there are victors and vanquished every one of our discoveries has evoked long and bitter fights. All were eventually decided in our favour’.
Somehow he could not resist asserting himself in this way, although it was quite unnecessary. How different he was in this respect from other scientists. Thus when in 1953 Einstein’s attention was drawn by Max Born to comments of Edmund Whittaker (who wrongly, in the view of most scientists, attributed the discovery of relativity to Lorentz and Poincare) replied ‘Do not worry. Everybody must behave according to what he thinks is right. If he convinces others it is their business. I found my own efforts very gratifying but I do not consider it a sound proposition to defend my few results as “property”. I am not at all angry with him. After all, I need not read the stuff’ (16).
Warburg, on the other hand, was liable to be angry and he accused his fellow scientists of being prejudiced and even dishonest. Dishonest because he believed that they clung to out-of-date ideas because it was dangerous to disagree with people in positions of power. Having read what Andrade (1) wrote in Notes and Records in 1964 about Galileo’s difficulties in getting recog nition, he commented that he, Warburg, had exactly the same kind of difficulties as Galileo, of whom Andrade wrote:
‘Besides his wonderful genius for scientific discovery by observation and experiment, which led to the replacement of the authority of the ancients by the methods of modern science, he had a notable gift which was the source of all his troubles: he was a master in the art of polemic and welcomed con troversy, which Newton so conspicuously shunned. It was his mordant and aggressive argument that led to his misfortunes. It is not in general dangerous
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
Otto Heinrich Warburg 667
to be apologetically, or just quietly right, but to be right and to insist causti cally and convincingly that you are right in the face of firmly established authority may be fatal, as Galileo found.’
Warburg’s own assessment of his work
In 1961, Warburg himself summarized his achievements and the reasons for his great successes in the introduction to a volume of his collected papers [508]. It is quoted here in full because it is a fair summing up, and apart from this it reveals other characteristics of his personality. It conveys his clear and forceful style of writing. It illustrates his tendency to over simplify complex issues and to make somewhat exaggerated claims when he writes ‘the de-differentiated growth of cancer cells was explained by a type of energy transformation at a time when the atmosphere of the earth did not yet contain oxygen—a result, which explains the ultimate cause of cancer by the anaerobic past of life’. And it is also evident that the summary was written by a man who did not hide his light under a bushel.
‘As a co-worker of Emil Fischer, I prepared the first optically active peptides in the years from 1903 to 1906, and thereby, as one who belonged to the inner circle of his co-workers, I came to know the methods and experimental techniques of this greatest organic chemist of our times. Later on I began work on the quantum requirement of photosynthesis in the radiation laboratory of the Physikalisch-Tech- nische Reichsanstalt, where my father Emil Warburg was president from 1906 to 1922. From the experiments of this laboratory, Max Planck derived his law of radiation and calculated “the quantum of action”, h = 6.55.10~27 erg.sec. In the same laboratory Emil Warburg measured the first quantum yields of photochemical reactions.
‘Thus it came about that in the Kaiser-Wilhelm-Institut fur Zell- physiologie the methods of two widely separated fields, those of radiation physics and organic chemistry, were united in one hand. The result was the elucidation of the chemical mechanism of enzyme action— the very fast intermediate reactions of protein-bound active groups, that we were able to separate from the enzymes and to crystallize. Coenzymes and many vitamins proved to be nothing else than free active groups of enzymes.
‘Here are some examples to illustrate the interplay of physical and chemical methods that is characteristic of our institute.
‘By the methods of radiation physics the substance was discovered, that reacts in the living world with the molecular oxygen. By the methods of organic chemistry this substance which is an iron compound was isolated and crystallized. By the methods of radiation physics the acting groups of the hydrogen-transferring enzymes were discovered, by the methods of organic chemistry these acting groups were separated
43A
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
668
Biographical Memoirs
and crystallized. From the interaction of the oxygen- and hydrogen transferring enzymes resulted the respiratory chain—the general solution of the problem of Lavoisier’s respiration. By the methods of radiation physics the main reaction of fermentations was recognized as a hydrogen transfer by nicotinamide, by the methods of organic chemistry 1,3-diphosphoglyceric acid has been isolated from yeast, the reactions of which explain the production of phosphate energy in fermentations. By methods of physics the fermentation of cancer cells was discovered and the de-differentiated growth of cancer cells was explained by a type of energy transformation at a time when the atmosphere of the earth did not yet contain oxygen—a result, which explained the ultimate cause of cancer by the anaerobic past of life.
‘The methods of radiation physics were employed to measure the quantum requirements of photosynthesis. It was found that in Chlorella somewhat less than three light quanta are necessary to split 1C 0 2 into C + 0 2; this means that in red light there is an almost complete con version of light energy into chemical energy. In this energy transforma tion 1 light quantum splits off 1 molecule of oxygen from the photolyte which is a carbonic acid, transformed by the energy of respiration; f of the produced C and 0 2 enter into a back reaction so that, in the net balance, three light quanta split one molecule of carbon dioxide. Thus the problem of the multiquanta reactions no longer exists in photo synthesis. The process of photosynthesis is part of the ordinary photo chemical reactions of the nonliving world. On this basis, the special chemistry of photosynthesis is nowadays being investigated with the methods of manometry and organic chemistry; our findings in general energetics have thereby found excellent confirmation.
‘The best physical and chemical methods, however, would not have been able to advance cell physiology, had they not been combined with
a simplification of the biological technique. Instead of measuring the
flow of blood through the surviving organs, the use of thin tissue slices
was introduced; instead of tissues, single cells were employed later. Red blood cells—nonnucleated ones of mammals, and nucleated ones of birds—were the first objects used to investigate the chemical mechanism
of cell respiration. Instead of green leaves employed to study photo synthesis, unicellular green algae were introduced; the algae were some times replaced by the green grana of spinach chloroplasts or the even much smaller green grana of lrehoC,produced waves. In the investigation of cancer cells, ascites cancer cells were substituted for tumours; whole animal cells were replaced by the grana
of liver cells as early as 1914. In all this experimentation it was always
our aim to simplify the technique and to attain a speed and precision comparable to the methods of volumetric chemical analysis. With complicated methods we have never discovered anything significant.’
In support of his fight for recognition of his views, Warburg liked to
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
OttoHeinrich Warburg 669
demonstrate his key experiments and in the early 1950s he set aside a special room for demonstrating the most important methods and discoveries, especially in the field of photosynthesis. His own description which stresses the historical perspective of his work runs as follows [370]:
‘Recently we set up in our laboratory a room in which some important methods and discoveries in the field of photosynthesis are demonstrated to everybody who is interested.
‘We demonstrate the use of the special bolometer which was developed by Lummer and Kurlbaum towards the end of the last century at the National Physical Laboratory at Charlottenburg, the same laboratory where in 1899 Lummer and Pringsheim measured bolometrically the radiation of black bodies and obtained the data from which Max Planck in 1900 calculated the value of the action quantum , where Emil Warburg—between 1915 and 1930—for the first time measured the energetics ofphotochemical reactions and where Otto Warburg—in
1920—for the first time measured the energetics of photosynthesis.
‘We also demonstrate our manometry by which we measure the effects of light on photosynthesis. It is the same manometry by which we discovered the oxygen-transferring iron of respiration, the yellow enzymes, nicotinamide and the mechanisms of biological hydrogen transfer, the oxidative reaction of fermentation and thus the chemical
mechanism of fermentation; in brief, a large part of the biochemistry of today. We demonstrate also the cultivation of a Chlorella which utilizes light maximally, methods without which even perfect bolometry and manometry are useless. In this field also, that of biological methods, we have the earliest and longest experience: it was Chlorella which we introduced in 1919 as experimental material for photosynthesis research; and since 1919 all relevant papers in this field have been carried out on Chlorella.
‘He who has learned in our laboratory the three essential methods— bolometry, manometry and cultivation of Chlorella—can carry out the most important experiments of photosynthesis himself; he can see how one quantum of light generates one molecule of oyxgen from Chlorella; how in the dark two-thirds or more of the oxygen evolved in the light back-reacts through respiration; how photosynthesis in monochromatic red light decreases and with blue-green light rises; he can measure the action spectrum of blue-green light and he can see and learn many other things.’
At a relatively early stage ofhis career, Warburg became fully aware ofhis outstanding ability. He knew that in his field of biological chemistry he towered above the great majority of even the leading scientists of his genera tion. He felt that he carried on where Pasteur (who, too, was primarily trained as a chemist and who applied his knowledge to biology) had left off. Well before he received a Nobel Prize he thought that he was due to one
43B
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
670 Biographical Memoirs
and so he thought that justice was done when it came—unlike many other laureates who, though conscious of their ability, would not place themselves above many other candidates.
Once when a colleague mentioned to him that he had taken part in a discussion on whether Warburg or Otto Meyerhof (his pupil) was the greater scientist, Warburg was taken aback and asked whether this was really a question requiring discussion. But a little while later, after some reflexion, he came back to the subject and commented ‘Meyerhof war vielleicht kliiger, aber ich konnte mehr’. This is a remarkably detached, reasonable and modest assessment.
Of his greater skill (‘Konnen’) there can be no doubt. Meyerhof’s tech nique in the laboratory was not particularly good, whilst Warburg was a genius in devising and handling techniques. Meyerhof’s intellectual interests may have covered a wider area, but I do not think his intelligence was greater. Warburg’s skill was not merely technical but highly intellectual. Of course their intelligences were not of the same kind.
Teaching and committee work
Warburg was not much interested in teaching and in educating subsequent generations of scientists. When a colleague once mentioned to him the im portance he attached to teaching and in particular to transmitting a tradition of attitudes to the scientists of the future, implying that this would be a worthwhile job for Warburg, he replied: ‘Look, Meyerhof, Theorell and Krebs were my pupils. Have I not done enough for the next generation?’ He never taught undergraduate courses. Occasionally he gave lectures but these were addressed to fellow scientists. He rarely attended scientific conferences and declined the great majority of lecture invitations because he deprecated the widespread practice of ‘academic tourism’. Accepting too many opportunities of travel, he said, is the productive scientist’s way Oi perishing honorably—but perish he will, especially the senior scientist.
He kept aloof from committee work, because he regarded much of it as a waste of time. After World War II, his advice was often sought and readily given, especially within the councils of the Max Planck Gesellschaft but he tended to be cross when his advice was not fully accepted and he restricted the time spent on such work to a minimum.
Eccentricities
Some of Warburg’s eccentricities concerned food. Once it was clear to him that cancer can arise from a large number of chemicals if they are applied for long periods, he became extremely careful in avoiding food which had been treated with special chemicals (‘additives’). He had heard that bakers tend to get eczema and he thought that this had something to do with the bleaching chemicals added to the bread. So he would never eat bread from
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
Otto Heinrich Warburg 671
a baker’s shop it he could help it. For the last fifteen years or so he insisted on eating only such bread as Heiss baked at home. Once a week Heiss had to spend one long evening baking; this he had learned at a farmhouse. The unadulterated flour was obtained from a country miller. Warburg installed in his house an electric baking oven and eventually also an electric kneading machine. He was also afraid of artificial fertilizers, of insecticides and pesticides. He enlarged his garden by buying adjacent allotment land increasing the size to 4500 square metres in order to produce food. He kept hens, ducks, geese, turkeys and rabbits and employed a full-time gardener to look after the animals and the garden. He grew most of the vegetables he needed, and also pears, apples, strawberries, raspberries, red currants and apricots. He would not permit the use of artificial fertilizers or of pesticides in his garden. From his horses and his poultry he got sufficient manure. To control pests he encouraged the nesting of tits. He had 10 nest boxes in his garden and he saw to it that the tits were well-fed during the winter so that they did not go away. During the summer season they were very diligent catching the caterpillars and other pests.
Through the School of Agriculture he obtained milk from a special herd. He arranged for the supply of 30 litre quantities at a time; these were centri fuged in the laboratory and converted into cream and butter. Occasionally he permitted the purchase of French butter because in France the use of herbicides and pesticides was more strictly controlled than in Germany. Altogether his whims, fancies and anxieties about food were at times rather exasperating to Heiss.
Another expression of his eccentricity was his somewhat exaggerated love for everything English. Ever since he first visited Cambridge before World War I he was an Anglophile. He loved England because, he once said ‘the English tolerate headstrong and eccentric (“eigensinnige”) people—such as me’. He also loved the adherence to tradition and the dignified pomp and circumstance of the ceremonial which he experienced when he was given an honorary degree at Oxford in 1965. ‘England is the last bastion of old Europe’, he said. Earlier in his life he travelled twice a year to England for brief periods to purchase suits in Savile Row, riding kit and above all antique furniture. He surrounded himself in his house with beautiful pieces of old English furniture. He held when something was English it could not be wrong. He was a long standing subscriber to the Times.
Weaknesses
Warburg’s exceptional qualities of intellect and character were liable to be influenced by deep emotional attitudes which tended on occasion to cloud hisjudgement. His intellect enabled him to approach his chosen field of work with sharp, constructive and critical reasoning and with wide-ranging imagination and resourcefulness. His character qualities enabled him to pursue his aims with dedication and discipline, and to avoid the distractions
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
672 Biographical Memoirs
which tempt successful scientists into the ‘corridors of power’, to travel and to waste time on being lionized.
Such qualities were the fount of his outstanding achievements because emotional factors did not enter his routine day-to-day research. Emotions came to the fore when his work—and this meant his conclusions, not his experiments—was criticized by others, or when he thought, often wrongly, that people did not treat him and his work with due respect. Then he was liable to be touchy and resentful, and he could not forget or forgive even minor quarrels. Many—including many of his admirers—suffered from this. He quite wrongly accused people of lack of intellectual honesty or simply
of incompetence, choosing to misinterpret a comment or to imagine a slight; and humility, the greatest of all balancers, was not at all his forte. All this, combined with an urge to assert himself, led sometimes to a failure to see other points of view and to the fruitless controversies. It was a failing which, alas, brought upon him an intellectual isolation, and so it came about that a distinguished biochemist, Efraim Racker (40), was prompted, not without
justification, to write in 1972 ‘Few will challenge the statement that Warburg was one ofthe great biochemists. His experimental approach was monumental and ingenious. Yet Warburg’s views on the two vital areas of his research interests, cancer and photosynthesis, are now almost universally dismissed as erroneous and naive’.
Such comments, it should be emphasized, refer to views, not to experiments He rightly prided himself that all his results were readily reproducible. The execution of his experiments was never influenced by wishful thinking though he could sometimes forget all about other people’s experiments and arguments if they did not suit his theoretical concepts. A weakness, then, was his passion for his own theories, a passion which blinded him on occasions.
One suspects that some of the eccentricities in his personal life had similarly their roots in judgements distorted by emotion. His food fads arose from exaggerated fears of cancer and some of his other attitudes from an over anxiety to do everything possible for his health.
Roots of Warburg's personality
Warburg’s personality was no doubt powerfully shaped by his family background—through both heredity and environment. Many of his ancestors were hard-working, self-disciplined, public-spirited and devoted to learning and other matters of the mind. Several of his close relations have also been described as strong individualists, self-contained and not readily forming warm personal friendships.
A contemporary relation, the art historian Aby Warburg, was character ized in obituaries by passages which would also fit his cousin Otto: ‘Un relenting against compromise and half-measures, a fighter, ajudge courageous and severe, a servant of scholarship. So powerful was his personality that he will live on as an inspiring example.’ Another writer thought that Leonardo
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
Otto Heinrich Warburg 673
da Vinci’s saying ‘He never turns back who has found his star’ was a fitting motto for Aby W arburg’s life. It also fits Otto.
His father was a model for Otto’s style of scientific writing aiming at maximum brevity and clarity as exemplified by Emil Warburg’s classical textbook. Emil was also his model for high standards of conduct and dedica tion, as may be gathered from what James Franck (18), one of the many distinguished pupils of Emil Warburg, said in an obituary address—the same
James Franck whom Otto Warburg later attacked in an acrimonious controversy: ‘Students who were not impelled by a powerful desire to learn and who were not prepared to put into research everything they had got knew that there was no point in asking Warburg to be accepted into his laboratory. But once accepted, one joined a community like a large “family” and one lived almost entirely in the laboratory. Members of the “family” appeared punctually early in the morning. The lunch interval—the time of the main meal—was brief and the evening meal was usually taken in the laboratory because work had to continue until late into the night.’ Of Emil’s style of writing, Franck said ‘His famous textbook of experimental physics was an example of brevity and clarity; every word mattered.’
Thefascination of Warburg'spersonality
Warburg made a deep impression on all who came into contact with him, irrespective of whether or not they approved of his views. Whenever people who knew him met, conversation sooner or later turned to the subject of his personality. What fascinated people was, apart from his penetrating intelligence which gave him a deep understanding of many aspects of his science and of affairs generally, his intellectual honesty and straight forwardness, his singularity of purpose and his industry, the wit and humour of his pertinent comments on affairs, scientific and other, his generosity in helping people in the laboratory and his oddities and extravagances. Those who knew him well were very ready to overlook his weaknesses, his touchi ness, his resentfulness, his prejudices and his harshness against those whom he regarded unjustifiably as his ‘enemies’. Thus David Keilin, despite having been rather abused and ridiculed, was keen to support Warburg’s nomina tion for the foreign membership of the Society.
Jacob Heiss
A special acknowledgement must be given to Warburg’s long-standing companion, Jacob Heiss, who after his demobilization in 1919 came to keep house for Warburg. Warburg was then looking for someone and Heiss was recommended by his military friends. Heiss remained a bachelor; he was born in 1899 and during World War I served in a Prussian infantry regiment of the Guards. Out of the initial servant-master relationship rapidly grew a close personal friendship. Warburg and Heiss became virtually inseparable
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
674 Biographical Memoirs
companions, spending not only the daily routine together but also their holidays and visits to entertainments. Later Heiss became unofficially secretary and manger of the Institute—unofficially in that until very late in his life he was not paid by the Institute, although he spent much time in its service. Heiss was an understanding, devoted and self-denying friend who did everything he could to support and protect Warburg—not always an easy companion—and to make his life as congenial as possible. He also did much in calming and appeasing Warburg’s emotions when they were raised by controversy and resentment. For though caring for Warburg most con siderately, Heiss would not hesitate to express his own independent and often shrewd views. Warburg valued his commonsense approach and accepted his contradiction.
H onours
The many honours bestowed upon Warburg include, apart from the Nobel Prize, Foreign Membership of the Royal Society (1934), an Honorary Doctorate of Oxford University (1965), the German Order of Merit (re stricted to 30 of Germany’s most distinguished citizens) and the Freedom of the city of Berlin. Quite a few of the honours which he was offered he felt unable to accept, among them an Honorary Doctorate of Newcastle Univer sity, because he did not wish to spare the time to have them conferred personally, a usual condition of honorary doctorates.
The book Nobel, the man and his Prizes, published by the Nobel Foundation (32), which gives glimpses of the work of the Nobel committees, records that no less than three times was Warburg considered worthy of a Nobel prize, each time on account of a different piece of work. In 1927 it was his work on the metabolism of cancer cells. The proposal was that the Prize should be divided with Fibiger but the Faculty preferred to give Fibiger the undivided Prize for his discovery of the Spiroptera carcinoma. Incidentally, the subsequent evaluation of Fibiger’s work cast grave doubts on the wisdom of this decision. In 1931 Warburg was awarded the Prize for his discovery of the catalytic role of iron porphyrins in biological oxidations. In 1944 he was again found to deserve the honour for the identification of the flavins and of nicotinamide as hydrogen carriers in biological oxidations, but Hitler’s decree forbidding the acceptance of Nobel Prizes by German citizens intervened.
Warburg was of course not alone in deserving more than one Prize. Emil Fischer, Ernest Rutherford, Albert Einstein, Max Born and others all had several achievements to their credit which deserved a Nobel Prize. Evidently there are scientists who maintain the highest standard of achievement over long stretches of their lives; whose Prizes are not merely a question of one lucky hit (as they may be in some cases) but an expression of sustained great ness in research.
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
OttoHeinrich Warburg 675 Concluding remarks
The rapid progress of science and the volume of material to be covered in textbooks and in teaching leaves little time for historical aspects. History has been crowded out and the new generation tends to be ignorant of earlier pioneers. Many of Warburg’s discoveries are now incorporated in the textbooks, often without reference to the originator.
Of Warburg the scientist it may be said, as Cuvier (11) said of Humphry Davy (when comparing him with other leading scientists); ‘He rose like an eagle and from high above illuminated a large area of science with a bright torch. Others modestly shone their little lights on limited objectives. His name stands at the head of many chapters; the names of the others appear in single paragraphs’.
Of Warburg the man it may be said, as Goethe said of Faust, he was one of those ‘who never ceased to strive’. And thus, like Faust, he was a good man.
Acknowledgments
Warburg never found the time to respond to the request of the Society to provide biographical information.
I am indebted to the following for help and information:
Family and friends: Jacob Heiss (companion); Gertrud von Wartenburg (sister); P. G. Meyer-Viol (nephew); Elisabeth Thalgott (niece); Professor Johannes Fuchs (second cousin); Paula Scholler (friend); Dr Margaret
Boveri; Dr Rudolf Gruber.
Scientific colleagues: M. von Ardenne; D. I. Arnon; H.-U. Bergmeyer; Th. Bucher; D. Burk; A. Butenaudt; J. N. Davidson; F. Dickens; H. Gaffron; K. Gawehn; B. Hess; R. Hill; G. Krippahl; S. Lorenz; F. Lynen; T. Terronova; H. H. Weber; F. H. Whatley.
Others: Frau Marie-Luise Rehder (Max-Planck-Gesellschaft); Frau K. Saupe (Max-Planck-Gesellschaft); Dr C. C. Worthington (Worthington Biochemical Corporation); Dr B. R. Cookson (Miles-Seravac Ltd.); B. von der Marwitz; K. F. Reimers (Institut fur den Wissenschaftlichen Film).
Special thanks are due to Professor D. I. Arnon, Dr R. Hill and Professor F. R. Whatley for help with the section on ‘Photosynthesis’; to Dr F. Reimers for permission to quote a transcript of the film interview of 1966; to Professor M. von Ardenne for a copy of Einstein’s letter to Warburg and Herr Jacob Heiss for information on numerous details.
H. A. KREBS
The photograph on the right (facing page 629) was made available by Jacob Heiss, that on the left by Willy Pragher of Freiburg im Breisgau.
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
676 Biographical Memoirs R eferences
(1) Andrade, E. N. da C. 1964. Galileo. Not. Rec. R. Soc. Lond. 19, 120-130.
(2) Arnon, D. I. 1969. Role of ferredoxin in photosynthesis. Naturwissenschaften, 56, 295-
305.
(3) Arnon, D. I. 1971. The light reactions of photosynthesis. Proc. Nat. Acad. Sci. USA. 68,
2883-2892.
(4) Barron, E. S. G. and Harrop, G. A. Jr., 1928. Studies on blood cell metabolism.
J. Biol. Chem. 79, 65-87.
(5) Bayliss, W. M. 1920. Principles ofgeneralphysiology, London; Longmans Green & Co.
(6) Beevers, L. & Hageman, R. H. 1969. Nitrate reduction in higher plants. Ann. Rev.
Plant Physiol. 20, 495-522.
(7) Broyer, T. C., Carlton, A. B., Johnson, G. M. and Stout, P. R. 1954. Chlorine—a
micro-nutrient element for higher plants. Plant Physiol. 29, 526-532.
(8) Burke, P. 1971. The Warburg tradition. The Listener, 21 October, 546-548. (An
appreciation of Aby Warburg).
(9) Chorine, V. 1945. Action de l’amide nicotinique sur les bacilles du genre Myco
bacterium. C. r. liebd. seanc. Acad. Sci. Paris, 220, 150-151.
(10) Claude, A. 1947-48. Studies on cells. Morphology, chemical constitution, and distribu
tion of biochemical functions. The Harvey Lectures, 43, 121-164.
(11) Cuvier, G. L. 1829. Eloge. Mem. Acad. R. Sci. ITnstit. deFrance, 12, LVI.
(12) Davenport, H. E., Hill, R. and Whatley, F. R. 1952. A natural factor catalysing
reduction of methaemoglobin by isolated chloroplasts. Proc. R. Soc. Lond. B, 139,
346-358.
(13) Earle, W. R. and Nettleship, A. 1943. Production of malignancy in vitro. V. Results
of injections of cultures into mice. J, Nat. Cancer Inst. 4, 213-227.
(14) Evans, V. J. and Andersen, W. F. 1966. Effect of serum on spontaneous neoplastic
transformations in vitro. J. Nat. Cancer Inst. 37, 247-249.
(15) Evans, V. J., Parker, G. A. and Dunn, Thelma B. 1964. Neoplastic transformations
in C3H mouse embryonic tissue in vitro determined by intraocular growth. 1. Cells from chemically defined medium with and without serum supplement. J. Nat. Cancer Inst. 32, 89-107.
(16) Einstein—Born Briefwechsel 1916-1955. Nymphenburger Miinchen: Verlagshandlung GmbH, 1969.
(17) Fick, A. 1893. Mechanisclie Arbeit und Warmeentwicklung bei der Muskelthatigkeit. Leipzig: Verlag Brockhaus.
(18) Franck, J. 1931. Emil Warburg zum Gedachtnis. Naturwissenschaften, 19, 993-997.
(19) Gey, G. O. 1941. Cytological and cultural observations on transplantable rat sarco mata produced by the inoculation of altered normal cells maintained in continuous
culture. Cancer Res. 1, 737.
(20) Goldblatt, H. and Cameron, G. 1953. Induced malignancy in cells from rat myo
cardium subjected to intermittent anaerobiosis during long propagation in vitro. J . Exp. Med. 97, 525-552.
(21) Haldane, J. S. and Smith, J. L. 1896. The oxygen tension in arterial blood. J . Physiol. 20, 497-520.
(22) Henri, V. 1926. Die spezifische photochemische Wirkung bei der Kohlensaureas- similation nach den Versuchen von Wurmser. Naturwissenschatten, 14, 165-167.
(23) Hess, B. 1963. Enzymes in blood plasma, (translated by K. S. Hensley). New York: Academic Press.
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
(24) (25) (26) (27)
(28) (29) (30)
(31) (32)
(33) (34)
(35) (36)
(37) (38)
(39)
(40)
(41)
(42) (43)
(44) (45)
(46) (47) (48) (49)
Otto Heinrich Warburg 677
Hill, R. 1965. The photosynthetic electrontransport in plants. in Biochemistry, 1, 121-151.
Keilin, D. 1966. The history of cell respiration and of cytochrome. Cambridge University Press.
Keilin, D. and Hartree, E. F. 1939. Cytochrome and cytochrome oxidase. Proc. R. Soc., .donLB, 127, 167-191.
Kleinzeller, A. (Editor). 1965. Manometrische Methoden. Jena: Verl. Gustav Fischer Verlag.
Krebs, H. A. 1972. Essays in Biochemistry, 8, 1.
Kroebel, W. 1964. Zum Tode von James Franck. 51, 421-423. Leloir, E. F. 1971. Two decades of research on the biosynthesis of saccharides. Science,
172, 1299-1303.
Lewis, G. N. and Randall, M. 1923. Thermodynamics and the free energy of chemical
substances. New York, Toronto, London: McGraw-Hill.
Liljestrand, G. 1950. The prize in physiology and medicine. In Nobel, the man and his
prizes. Stockholm: Sohlmans Forlag.
Mann, T. 1964. David Keilin, Biog. Mem. R. Soc., Lond. 10, 183-205.
Martin, G. and Lavolley, J. 1958. Le chlore, oligo-^l^ment indispensable pour Lemna
minor. Experientia. 14, 333.
Mortenson, L. E., Valentine, R. C. and Carnahan, J. E. 1962. An electron transport
factor from Clostridium pasteurianum. Biochem. Biophys. Res. Commun., 7, 448-452.
Niel, C. B. Van. 1962. The present status of the comparative study of photosynthesis.
Ann. Rev. Plant Physiol. 13, 1-26
Panofsky, E. 1930. In a privately printed pamphlet ‘ auf Aby ’. Planck, M. 1950. A scientific autobiography, (translated by F. Gaynor.) London:
Williams & Norgate Ltd., p. 33.
Pullman, M. E., San Pietro, A. and Colowick, S. P. 1954. On the structure of reduced
diphosphopyridine nucleotide. J. Biol. Chem., 206, 129-141.
Racker, E. 1972. Bioenergetics and the problem of tumor growth. Amer. Scientist. 60,
56-63.
Reichert, I. 1938. Otto Warburg. Palestine J. Botany, (Rehovot Series) 2, 2-16. (An
appreciation of the botanist Otto Warburg).
Slater, E. C. 1964. In memoriam David Keilin. Enzymologia, 26, 131-320.
Stern, K. G. and Holiday, E. R. 1934. Die Photoflavine, eine Gruppe von Alloxazin-
Derivaten. Ber. deutsch. Chem. Ges. 67, 1442-1452.
Warburg, E. 1930. In a privately printed volume ‘Aby M. Warburg zum Gedachtnis'. Warburg, F. S. 1914. Die Geschichte der Firma R. D. Warburg, ihre Teilhaber und deren
Familien. Berlin: Privately printed by Otto Dreyer, Berlin W.57. (Available at
Deutsche Staatsbibliothek, 108 Berlin, Postfach 1312, DDR).
Wechsler, J. 1966. Profile: A Prince of the City, New Yorker, 9 April, 45-77. (A
‘Profile’ of Siegmund Warburg).
Weinhouse, S. 1956. Oxidative metabolism of neoplastic tissue. Science, 124, 267-
269.
Willstatter, R. 1926. Uber Fortschritte in der Enzym-Isolierung. Ber. deutsch. chem.
Ges. 59, 1-12.
Willstatter, R. 1958. Aus meinem Leben. Zweite Auflage. Weinheim: Verlag Chemie,
p. 200.
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
678 Biographical Memoirs BIBLIOGRAPHY
The list of publications contains a number of papers from Warburg’s laboratory published without his name. These papers were included because Warburg himself was a major contributor to the work. He was generous in giving credit to his juniors.
The bibliography, though extensive, is not complete. It was the intention to include all essential papers.
[1] 1905.
[2] 1905.
[3] 1905.
[4] 1905.
[5] 1906.
[6] 1908.
[7] 1909.
[8] 1909.
[9] 1909.
[10] 1910.
[11] 1910.
[12] 1910.
[13] 1910.
[14] 1910.
[15] 1911.
[16] 1911.
[17] 1911.
[18] 1911.
[19] 1911.
[20] 1911.
[21] 1912.
[22] 1912.
Spaltung des Leucinesters durch Pankreasferment. chem. Ges. 38, 187. (With E. F is c h e r ). Spaltung des Leucins in die optisch-aktiven Komponen-
ten mittels der Formylverbindung. Ber. dt. chem. Ges. 38, 3997. (WithE.Fischer).SynthesevonPolypeptiden.Glycyl-leucin,Alanyl-leucin, Leucyl-alanin, Glycyl-alanyl-leucin und aktives Alanyl-glycin. Justus
Liebigs Annin. Chem. 340, 152.
(WithE.Fischer). Optischaktivea-Brompropionsaure.JustusLiebigsAnnin.
Chem. 340, 168.
Spaltung des Leucinesters durch Pankreasferment. J. physiol.
Chem. 48, 205.
Beobachtungen iiber die Oxydationsprozesse im Seeigelei. Hoppe-Seyler's J.
physiol. Chem. 57, 1.
Zur Biologie der roten Blutzellen. Hoppe-Seyler's J. physiol. Chem. 59, 112. Liber die Oxydationen im Ei. Hoppe-Seyler''s J. physiol. Chem. 60, 443. Massanalytische Bestimmung kleiner Kohlensauremengen. Hoppe-Seyler's J.
physiol. Chem. 61, 261.
tJber die Oxydationen in lebenden Zellen nach Versuchen am Seeigelei.
Hoppe-Seyler's J. physiol. Chem. 66, 305.
tJber Beeinflussung der Oxydationen in lebenden Zellen nach Versuchen an
roten Blutkorperchen. Hoppe-Seyler's J. physiol. Chem. 69, 452.
Bemerkung zu einer Arbeit von Jacques Loeb und Wasteneys. Hoppe-Seyler's
J. physiol. Chem. 69, 496.
(With O. N e u b a u e r ). Uber eine Synthese mit Essigsaure in der kiinstlich
durchbluteten Leber. Hoppe-Seyler's J . physiol. Chem. 70, 1.
tJber die giftige Wirkung der Natriumchloridlosung. Bioch. 29, 414.
tJber Beeinflussung der Sauerstoffatmung. Hoppe-Seyler's J . physiol. Chem. 70,
413.
(M. O n a k a .) Uber die Wirkung des Arsens auf die roten Blutzellen. Hoppe-
Seyler's J. physiol. Chem. 70, 433.
(M. O n a k a .) tJber Oxydationen im Blut. Hoppe-Seyler's J . physiol. Chem.
71, 193.
Uber Beeinflussung der Sauerstoffatmung. Hoppe-Seyler's Z- physiol. Chem. 71,
479.
Beziehungen zwischen Konstitution und physiologischer Wirkung. Verh. d.
Dtsch. Kongr.f. innere Med. 28, 553.
Untersuchungen uber die Oxydationsprozesse in Zellen. Munch, med. Wschr.
58, 289.
Untersuchungen liber die Oxydationsprozesse in Zellen (II). Munch, med.
Wschr. 59, 2550.
Uber Hemmungen der Blausaurewirkung in lebenden Zellen. Hoppe-Seyler's
Z-physiol. Chem. 76, 331.
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
[23] 1912.
[24] 1912.
[25] 1912.
[26] 1912.
[27] 1912.
[28] 1912.
[29] 1912.
[30] 1912.
[31] 1912.
[32] 1913.
[33] 1913.
[34] 1913.
[35] 1914.
[36] 1914.
[37] 1914.
[38] 1914.
[39] 1914.
[40] 1914.
[41] 1915.
[43] 1919.
[44] 1920.
[45] 1920.
[46] 1921.
OttoHeinrich Warburg 679
Notiz liber Bestimmung kleiner, im Wasser geloster C02-Mengen. Seyler's Z-physiol. Chem. 81, 202.
(R. Usui.) Uber die Bindung von Thymol in roten Blutzellen. Z~physiol. Chem. 81, 175.
(With R. W ie s e l .) Uber die Wirkung von Substanzen homologcr Reihen auf Lebensvorgange. PJliiger’s Arch. ges. Physiol. 144, 465.
Uber Beziehungen zwischcn Zellstruktur und biochemischen Reaktionen. I. PJliiger’s Arch. ges. Physiol. 145, 277.
(With O . M e y e r h o f .) Uber Atmung in abgetoteten Zellen und in Zell- fragmenten. PJluger’sArch. ges. Physiol. 148, 295.
(E. G r a f e .) Uber die Wirkung von Ammoniak und Ammoniakderivaten auf
die Oxydationsprozesse in Zellen. popHZ- physiol. Chem.
(A. D o r n e r .) Uber Beeinflussung der alkoholischen Garung in der Zelle und
im Zellpressaft. Hoppe-Seyler’s Z - physiol. Chem. 81, 99.
(R. W i e s e l .) Uber die Wirkung von Blutserum auf die Oxydationsprozesse von Baktericn. sJ.f.S. Immunitatsforch. 12, 194.
(R. Usui.) Uber Messung von Gewebsoxydationen vitro. Arch. res. Physiol. 147, 100.
(A. D o r n e r .) Uber Titration kleiner Kohlensauremengen. Hoppe-Seyler’s physiol. Chem. 88, 425.
Uber sauerstoffatmende Kornchen aus Leberzcllen und liber Sauerstoffat- mung in Berkefeld-Filtraten wassriger Leberextrakte. PJluger’s Arch. ges. Physiol. 154, 599.
Antwort auf vorstchende Bemerkung Thunbcrgs. Hoppe-Seyler’s Z- physiol. Chem. 87, 83.
Uber die Rolle des Eisens in der Atmung des Seeigeleis nebst Bemerkungen liber einige durch Eisen beschleunigten Oxydationen. Hoppe-Seyler’s Z- physiol. Chem. 92, 231.
Uber Verbrennung der Oxalsiiure an Blutkohle und die Hcmmung dieser Reaktion durch indifferente Narkotika. PJluger’s Arch. ges. Physiol. 155, 547. Uber die Empfindlichkeit der Sauerstoffatmung gegeniiber indilferenten
Narkotika. PJliiger’s Arch. ges. Physiol. 158, 19.
Zellstruktur und Oxydationsgeschwindigkeit nach Versuchen am Secigelci.
PJluger’s Arch. ges. Physiol. 158, 189.
(A. D o r n e r .) Uber Vertcilungsgleichgewichte einiger indifferenter Narkotika
Sitzungsberichte Sber. Heidelberg. Akad. Wiss. Math.-Nat. Klasse Abt. B. 1.
Abhandlung, 3.
Beitrage zur Physiologie der Zelle, insbesondere liber die Oxydationsgeschwin
digkeit in Zellen. Ergebn. Physiol. 14, 253.
Notizen zur Entwicklungsphysiologie des Seeigeleis. PJluger’s Arch. ges.
Physiol. 160, 324.
Uber die Geschwindigkeit der photochemischen Kolilensaurezersetzung in
lebenden Zellen. Biochern. Z- 100, 230.
Uber die Geschwindigkeit der photochemischen Kohlensaurezersetzung in
lebenden Zellen II. Biochem. Z•103, 188. (With E. N e g e l e i n .) Uber die Reduktion der Salpetersaure in grlinen
Zellen. Biochem. Z - HO, 66.
(With E. N e g e l e i n .) Uber die Oxydation des Cystins und andercr Amino-
sauren an Blutkohlc. Biochem. Z- 113, 257.
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
6 8 0
Biographical Memoirs
[47] 1921.
[48] 1921.
[49] 1922.
[50] 1922.
[51] 1922.
[52] 1923.
[53] 1923.
[54] 1923.
[55] 1923.
[56] 1923.
[57] 1923.
[58] 1923.
[59] 1923.
[60] 1923.
[61] 1924.
[62] 1924.
[63] 1924.
[64] 1924.
[65] 1924.
[66] 1924.
[67] 1924.
[68] 1924.
[69] 1925.
[70] 1925.
[71] 1925.
[72] 1925.
[73] 1925.
[74] 1925.
[75] 1925.
Physikalische Chemie der Zellatmung. Biochem. H9, 134.
Theorie der Kohlensaureassimilation.
(WithE.Negelein.) tjberdenEnergieumsatzbeiderKohlensaureassimila
tion. Z- Electrochem. 28, 449.
t)ber Oberflachenreaktionen in lebenden Zellen. Z- Electrochem. 28, 70. (With E. N e g e l e in .) tjber den Energieumsatz bei der Kohlensaureas
similation. Z •phys. Chem. 102, 235.
(WithE.Negelein.) tjberdenEinflussderWellenlangeaufdenEnergieum
satz bei der Kohlensaureassimilation. Chem. 106, 191.
(With E. N e g e l e i n .) Bemerkung zu einem Aufsatz von F. Weigert. • Phys.
Chem. 108, 101.
(WithS. Sakuma.) tjber diesogenannteAutoxydationdesCysteins.Pfluger’s
Arch. ges. Physiol. 200, 203. ,
Versuche an uberlebendem Carcinomgewebe. Biochem. Z- 142 , (S. M in a m i.) Versuche an uberlebendem Carcinomgewebe. Biochem. Z- 142
334.
(E.Negelein.) tjberdieReaktionsfahigkeitverschiedenerAminosaurenan
Blutkohle. Biochem. Z- 142, 493.
tjber die Grundlagen der Wielandschen Atmungstheorie. Biochem. 142,
518.
(With W. Brefeld.) tjber die Aktivierung stickstoffhaltiger Kohlen durch
tjber die antikatalytische Wirkung der Blausaure. Biochem. Z- 136 142, 68. ,
266.
(S. Sakuma.) tjber die sogenannte Autoxydation des Cysteins. Biochem. Z-
Eisen. Biochem. Z - (With M. Y a b u s o e .)
Biochem.
(With T. U y e s u g i.) tjber die Blackmansche Reaktion. Biochem. Z - 146, 486. Verbesserte Methode zur Messung der Atmung und Glykolyse. Biochem. Z-
152, 51.
Bemerkung liber das Kohlemodell. Biochem. • 132, 191.
(WithK.Posener andE.Negelein.) UberdenStoffwechselderCarcinom-
zelle. Biochem. Z- 152, 309.
tjber Eisen, den sauerstoffiibertragenden Bestandteil des Atmungsferments.
Biochem. Z• 152, 479.
(M. Y a b u s o e .) Uber den Temperaturkoeffizienten der Kohlensaureassimila
tion. Biochem. Z- 152, 498.
(M. Yabusoe.) tjber Eisen- und BlutfarbstofFbestimmungen in normalen
Geweben und in Tumorgewebe. Biochem. Z - 157, 388.
(K. T a n a k a .) Versuche zur Priifung der Wielandschen Atmungstheorie.
Biochem. Z• 137, 425.
(E. N e g e l e i n .) Versuche uber Glykolyse. Biochem. Z - 138, 121.
(F. W i n d .) t j b e r die Oxydation von Dioxyaceton und Glycerinaldehyd in
Phosphatlosungen und die Beschleunigung der Oxydation durch Schwer-
metalle. Biochem. Z 1• 39, 58.
(Y. O kamoto.) tjber Anaerobiose von Tumorgewebe. Biochem. Z- 160, 52. tjber Milchsaurebildung beim Wachstum. Biochem. Z - 160, 307.
Bemerkung zu einer Arbeit von M. Dixon and S. Thurlow sowie zu einer Arbeit von G. Ahlgren. Biochem. Z- 163, 252.
145, 461.
Uber die Oxydation von Fructose in Phosphatlosungen.
Z• 146, 380.
317
N9, 354
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
[76] 1925.
[77] 1925.
[78] 1925.
[79] 1925.
[80] 1925.
[81] 1925.
[82] 1925.
[83] 1926.
[84] 1926.
[85] 1926.
[86] 1926.
[87] 1926.
[88] 1926.
[89] 1926.
[90] 1926.
[91] 1926.
[92] 1927.
[93] 1926.
[94] 1926.
[95] 1926.
[96] 1926.
[97] 1926.
[98] 1926.
[99] 1926.
[100] 1926.
[101] 1926.
[102] 1926.
[103] 1927.
[104] 1927.
OttoHeinrich Warburg 681
(E. N e g e l e i n .) lib e r die Wirkung des Schwefelwasserstoffs auf chemische Vorgange in Zellen. Biochem. Z-165, 203.
Manometrische Messung des Zellstoffwechsels in Serum. Biochem. • 164, 481.
(E. N e g e l e i n .) t ) b e r die glykolytische Wirkung des embryonalen Gewebes. Biochem. Z 1• 65, 122.
t)ber die Wirkung der Blausaure auf die alkoholische Garung. Biochem. Z - 165, 196.
Versuche iiber die Assimilation der Kohlensaure. Biochem. Z - 166, 386. (H.Gaffron.) libereinephotochemischeWirkungdesHamatoporphyrins.
Naturwissenschaffen,13, 859.
t)ber Eisen, den sauerstoffubertragenden Bestandteil des Atmungsferments.
Chem. Ber. 58, 1001.
(M.Yabusoe.) liber HemmungderTumorglykolysedurchAnilinfarbstoffe.
Biochem. Z•168, 227.
(S. T o d a .) Uber die Oxydation der Oxalsaure durch Jodsaure in wasseriger
Losung. Biochem. Z - 171, 231.
(S. T o d a .) liber die Wirkung von Blausaureathylester (Athylcarbylamin) auf
Schwermetallkatalysen. Biocehm. Z - 172, 17.
(S. T o d a .) t)ber “ Wasserstoffaktivierung” durch Eisen. Biochem. Z - 172, 34. tiber die Wirkung von Blausaureathylester (Athylcarbylamin) auf die
Pasteursche Reaktion. Biochem. Z - 172, 432.
lib e r die Oxydation der Oxalsaure durch Jodsaure. Biochem. • 174, 497. liber die Wirkung des Kohlenoxyds auf den Stoffwechsel der Hefe. Biochem.
Z - 177,471.
(F. W in d .) Versuche liber den Stoffwechsel von Gewebsexplantaten und
deren Wachstum bei Sauerstoff- und Glucosemangel. Biochem. Z - 179, 384. (H. Gaffron.) liber Photoxydationen mittels fluoreszierender Farbstoffe.
Biochem. Z - 179, 157.
(H. A. K r e b s .) lib e r die Rolle der Schwermetalle bei der Autoxydation von
Zuckerlosungen. Biochem. Z- 180, 377.
Atmungstheorie und Katalase. Chem. Ber. 59, 739.
Uber die Wirkung von Kohlenoxyd und Licht auf den Stoffwechsel der Hefe.
Naturwissenschaffen, 14, 471.
Erwiderung auf einen Aufsatz von Henri. Naturwissenschaften, 14, 167.
(E. N e g e l e i n .) Uber Abtotung von Tumorzellen im Korper. Naturwissen
schaften, 14, 439.
liber kiin stliche Mineralwasser. Klin, wschr. 5, 734.
(WithF. Wind andE.Negelein.) liberdenStoffwechselvonTumorenim Korper. Klin. Wschr. 5, 829.
(With O. St a h l .) liber Milchsauregarung eines menschlichen Blasencarci- noms. Klin. Wschr. 5, 1218.
(F. W in d .) Versuche mit explantiertem Roussarkom. Klin. Wschr. 5, 1355. liber Carcinomversuche. Klin. Wschr. 5, 2119.
Das Carcinomproblem. Z- Angew.Chem. 39, 949. Uber den heutigen Stand des Carcinomproblems. Naturwissenschaften, 15, 1. Physiol. 8, 519.
(F.Wind andE.Negelein.) Themetabolismoftumorsinthebody.J. Gen. Physiol.
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
682
[105] 1927.
[106] 1927.
[107] 1927.
[108] 1927.
[109] 1927.
[110] 1927.
[111] 1927.
[112] 1927.
[113] 1927.
[114] 1927. [115] 1927.
[116] 1927. [117] 1927.
[118] 1927. [119] 1927.
[120] 1927. [121] 1927.
[122] 1927. [123] 1927. [124] 1927. [125] 1927. [126] 1928. [127] 1928. [128] 1928. [129] 1928. [130] 1928. [131] 1928.
Biographical Memoirs
(W.Fleischmann andF.Kubowitz.) liber denStoffwechselderLeucocyten. Biochem. Z - 181, 395.
Uber die Klassifizierung tierischer Gewebe nach ihrem Stoffwechsel. Biochem. Z- 184,484.
Uber den Stoffwechsel der Leucocyten. Biochem. • 187, 1.
Methode zur Bestimmung von Kupfer und Eisen und liber den Kupfergehalt
des Blutserums. Biochem. Z - 187, 255.
(H. A. K rebs.) liber den Stoffwechsel der Netzhaut. Biochem. Z- 189, 57.
(C. T a m iy a ). tiber den Stoffwechsel der Netzhaut in verschiedenen Stadien
ihrer Entwicklung. Biochem. Z•189, 114.
(C. T amiya). liber den Stoffwechsel der Leber in verschiedenen Stadien
ihrer Entwicklung. Biochem. Z ■189, 175.
(H.A.Krebs andF.Kubowitz). liberdenStoffwechselvonCarcinomzellen
in Carcinomserum und Normalserum. Biochem. Z - 189, 194. (WithF.Kubowitz.) StoffwechselwachsenderZellen(Fibroblasten,Herz,
Chorion). Biochem. Z •189, 242.
tiber den Stoffwechsel der Hefe. Biochem. Z- 189, 350.
liber die Wirkung von Kohlenoxyd und Stickoxyd auf Atmung and Garung.
Biochem. Z - 189, 354.
(With H. A. K r e b s .) tiber locker gebundenes Kupfer und Eisen im Blutserum.
Biochem. Z - 190, 143.
(G.Endres andF.Kubowitz.) StoffwechselderBlutplattchen.Biochem.Z-
191, 395.
tiber Kupfer im Blutserum des Menschen. Klin. Wschr. 6, 109.
(A. F u jit a .) tiber den Stoffwechsel der weissen Blutzellen. Klin. Wschr. 7,
897.
Milchsauregarung der Tumoren. .linKWschr. 6, 2047. (W. K e m p n e r .) Atmung im Plasma Pestkranker Hiihner. Klin. Wschr. 6,
2386.
tiber reversible Hemmung von Garungsvorgangen durch Stickoxyd. Natur-
wissenschaften, 15, 51.
tiber Kohlenoxydwirkung ohne Hamoglobin und einige Eigenschaften des
Atmungsferments. Naturwissenschaften, 15, 546.
(H. G a f f r o n .) Sauerstoffiibertragung durch Chlorophyll und das photo-
chemische Aquivalent-Gesetz. Chem. Ber. 60, 755.
(H. G a f f r o n .) Die photochemische Bildung von Peroxyd b e i der Sauerstoff-
tibertragung durch Chlorophyll. Chem. Ber. 60, 2229.
(W. C r e m e r .) Notiz uber die Reduktion von Hamin durch Cystein. Biochem.
Z. 192,426.
(S. K u m a n o m id o .) Stoffwechsel embryonaler Gewebe in Serum. Biochem. Z -
193, 315.
(With E. N egelein.) liber die Verteilung des Atmungsferments zwischen
Kohlenoxyd und Sauerstoff. Biochem. Z - 193, 334.
(WithE.Negelein.) tiber denEinflussderWellenlangeaufdieVerteilung
des Atmungsferments. Biochem. Z •198, 339.
(H. A. K r e b s .) tiber die Wirkung von Kohlenoxyd und Licht auf Hamin-
katalysen. Biochem. Z•193, 347.
(W. C r e m e r .) t i b e r eine Kohlenoxydverbindung des Ferrocysteins und
ihreSpaltungdurchLicht.Biochem.Z- 194,231.
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
[132]
1928.
1928. 1928.
1928. 1928.
1928. 1928.
1928. 1928.
1928. 1928. 1928. 1928. 1928.
1928.
1928. 1929.
1929. 1929. 1929.
1929. 1929.
1929. 1929.
1929.
Otto Heinrich Warburg 683
(F.Wind andK.v. Oettingen.) MilchsaurebestimmungindenUterus-und Nabelgefassen. Biochem. Z- 197, 170.
(A. Fujita.) tjber den Stoffwechsel der Korperzellen. Biochem. Z- 197, 175. (A. F u j i t a .) Uber die Wirkung des Kohlenoxyds auf den Stoffwechsel der
weissen Blutzellen. Biochem. Z- 197, 189.
Dber die oxydationskatalytische Wirkung des Eisens nach Handovsky.
Biochem.Z- 198, 241.
(With E. N e g e l e in .) Dber die photochemische Dissoziation von Eisencar-
bonylverbindungen (Kohlenoxyd-Hamochromogen, Kohlenoxyd-Ferro-
cystein) und das photochemische Aquivalentgesetz. Biochem. Z- 200, 414. (H. A. K r e b s .) Dber die Wirkung des Kohlenoxyds auf Hamatinkatalysen
nach M. Dixon. Biochem. Z•201, 489.
(W. C r e m e r .) Dber Hemmung der Ferrocysteinkatalyse durch Kohlenoxyd.
Biochem. -Z201, 490.
Wie viele Atmungsfermente gibt es? Biochem. Z- 201, 486.
(With E. N e g e l e in .) Dber die photochemische Dissoziation bei inter-
mittierender Belichtung und das absolute Absorptionspektrum des
Atmungsferments. Biochem. Z- 202, 202.
(With F. K u b o w i t z .) Notiz uber manometrische Messung kleiner Sauer-
stoffpartialdrucke. Biochem. Z- 202, 387.
(With F. K u b o w it z .) Dber die Konzentration des Fermenteisens in der
Zelle. Biochem. Z- 203, 95.
(M.Nakashima.) StofFwechselderFischnetzhautbeiverschiedenenTempera-
turen. Biochem. Z •204, 479.
Dber die chemische Konstitution des Atmungsfermentes. Naturwissenschaften,
16, 345.
(With E. N e g e l e i n .) Dber die photochemische Spaltung einer Eisencarbonyl-
verbindung und das photochemische Aquivalentgesetz. Naturwissenschaften,
16, 387.
Photochemie der Eisencarbonylverbindungen und das absolute Absorptions-
spektrum des Atmungsferments. Naturwissenschaften, 16, 856.
Stoffwechsel der Karzinomzelle. Verdlg. dtsch. Kongr. f . innere Med. 40, 11. (With E. N e g e l e i n .) Absolutes Absorptionsspektrum des Atmungsferments.
[133] [134]
[135] [136]
[137] [138]
[139] [140]
[141] [142] [143] [144] [145]
[146]
[147] [148]
[149] [150] [151]
[152] [153]
[154] [155]
[156]
Biochem. Z- 204, 495.
(H. A . K r e b s .) Dber die Wirkung von Kohlenoxyd und
Hamatinkatalysen. Biochem.
(H. A. K r e b s .) Dber die Wirkung der Schwermetalle auf die Autoxydation
der Alkalisulfide und des Schwefelwasserstoffs. Biochem. Z- 204, 343. (F.Kubowitz.) StoffwechselderFroschnetzhautbeiverschiedenenTempera- turen und Bemerkung uber den Meyerhofquotienten bei verschiedenen
Temperaturen. Biochem. Z 2• 04, 475.
1st die aerobe Glykolyse spezifisch fur die Tumoren? Biochem. Z- 204, 482. (W . C r e m e r .) Reaktionen des Kohlenoxyds mit Metallverbindungen des
Cysteins. Biochem. Z•206, 228.
Bemerkung zu einer Arbeit von D. Keilin. Biochem. 207, 494.
(H. A. K r e b s .) Dber Hemmung einer Hamatinkatalyse durch Schwefel-
wasserstoff. Biochem. Z- 209, 32.
(H. A. K rebs and J. F. D onegan.) Manometrische Messung der Peptid-
spaltung. Biochem. Z • 210, 7.
Blausaure auf
Z•204, 322.
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
684
[157] [158] [159]
[160] [161] [162]
[163]
[164]
[165]
[166]
[167]
[168]
[169]
[170]
[171]
[172]
[173] [174]
[175]
[176] [177] [178]
[179] [180] [181]
[182]
1929. 1929. 1929.
1929. 1929. 1929.
1929.
1929.
1929.
1929.
1930.
1930.
1930.
1930.
1930.
1930.
1930. 1930.
1930.
1930. 1930. 1930.
1930. 1931. 1931.
1931.
Biographical Memoirs Atmungsferment und Oxydasen. Biochem. 214, 1.
Atmungsferment und SauerstofFspeicher. Biochem. 214, 4.
(With F. K u b o w it z .) Atmung bei sehr kleinen Sauerstoffdrucken. Biochem. Z-
214, 5.
(With F. K u b o w i t z .) 1st die Atmungshemmung durch Kohlenoxyd voll-
standig? Biochem. Z• 214, 19.
(With F. Kubowitz.) Wirkung des Kohlenoxyds auf die Atmung von
Aspergillus oryzae. Biochem. Z-214, 24. (WithE.Negelein andW.Christian.) FiberCarbylamin-Hamoglobinund die photochemische Dissoziation seiner Kohlenoxydverbindung. Biochem. •
214, 26.
(WithE.Negelein.) FiberdasAbsorptionsspektrumdesAtmungsferments.
Biochem. Z- 214, 64.
(With E. N e g e l e in .) Fiber das Absorptionsspektrum des Atmungsferments
der Netzhaut. Biochem. Z•214, 101.
(WithF. Kubowitz.) Fiber Atmungsferment im Serum erstickter Tiere.
Biochem. Z- 214, 107.
Fiber die chemische Konstitution des Atmungsferments Elektrochem. 35,
549.
(H. A. K r e b s .) Manometrische Messung des Kohlensauregehaltes von
Gasgemischen. Biochem. Z- 220, 250.
(H.A.Krebs.) ManometrischeMessungderfermentativenEiweissspaltung.
Z •20, 283.
Versuche iiber die proteolytische Wirkung des Papains.
Z•220, 289.
(With F. K ubowitz and W. Christian.) Kohlenhydratverbrennung durch
Methamoglobin. Biochem. Z- 221, 494.
(H. L. A l t .) Fiber die Atmungshemmung durch Blausaure. Biochem. Z- 221,
498.
(H. Hartmann.) FiberdasVerhaltenvonKohlenoxydzuMetallverbindun-
gen des Glutathions. Biochem. Z 2• 23, 489.
(WithE.Negelein andE.Haas.) Spirographishamin.Biochem.Z-227,171. (WithF.Kubowitz.) FiberkatalytischeWirkungvonBluthaminenundvon
Chlorophyll-Haminen. Biochem. Z- 227, 184.
(WithF.Kubowitz andW.Christian.) FiberdiekatalytischeWirkungvon
Methylenblau in lebenden Zellen. Biochem. Z- 227, 245.
Notiz liber den Stoffwechsel der Tumoren. Biochem. Z- 228, 257.
(A. R e i d .) Oxydation von Leuko-Methylenblau. Biochem. Z- 228. 487.
(With E. N e g e l e i n .) Grimes Haemin aus Blut-Haemin. Ber. dt. chem. Ges. 63, 1816.
(A. R e i d .) Fiber die Oxydation scheinbar autoxydabler Leukobasen durch molekularen Sauerstoff. Ber. dt. chem. Ges. 63, 1920.
Fiber Nicht-Hemmung der Zellatmung durch Blausaure. Biochem. Z- 231, 493.
(With F. K ubowitz and W. Christian.) Fiber die Wirkung von Phenyl- hydrazin und von Phenylhydroxylamin auf die Atmung roter Blutzellen. Biochem. Z- 233, 240.
Wirkung der Blausaure auf die katalytische Wirkung des Mangans. Biochem. Z2.33, 245.
Biochem.
(H. A. K r e b s .) Biochem.
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
[183]
[184] [185]
[187] [188] [189]
[190]
[191]
[192] [193]
[194] [195]
[196] [197] [198] [199]
[200]
[201]
[202]
[203]
[204]
[205]
[206]
[207] [208]
[209]
1931.
1931. (WithW.Christian.) liberPhaohaminb.Biochem. 235,240.
Otto Heinrich Warburg 685
(With E. Negelein.) tlber die Hauptabsorptionsbanden der MacMunn- schen Histohamatine. Biochem. Z - 233, 486.
1931.
1931.
1931. (A.Reid.) ManometrischeMessungdersauerstofflosenAtmung.Biochem.Z-
242, 159.
1931. (With F. Kubowitz and W. Christian.) t)ber die Wirkung von Phenyl-
hydrazin und Phenylhydroxylamin auf den Stoffwechsel der roten Blut
zellen. Biochem. Z•242, 170.
1931. (With W. Christian.) t)ber Aktivierung der Robinsonschen Hexose-
Monophosphorsaure in roten Blutzellen und die Gewinnung aktivierender
(W. C h r i s t i a n .) Aktivierung von Kohlehydrat in roten Blutzellen. Biochem. Z - 238, 131.
(With E. N e g e l e i n .) Photographische Abbildung der Hauptabsorptions banden der MacMunnschen Histohamatine. Biochem. 238, 135.
Fermentlosungen. Biochem. Z - 242, 206.
1931. (With A. R e i d .) Oxydation von Hamoglobin durch Methylenblau. Biochem.
z 242, 149.
1931. Phaophorbid-b-Eisen. Ber. .tdchern. Ges. 64, 682. 1931. (E. N e g e l e i n .) Verbrennung von Kohlenoxyd zu Kohlensaure durch grime
und mischfarbene Hamine. Biochem. Z - 243, 386.
1931. (With E. N e g e l e i n .) Notiz liber Spirographishamin. Biochem. 244, 239. 1932. (With E. N e g e l e in .) Uber das Hamin des sauerstoffubertragenden Ferments
der Atmung, iiber einige kiinstliche Hamoglobine and liber Spirographis-
Porphyrin. Biochem. Z •244, 9.
1932. (With E. N e g e l e i n .) Notiz liber Spirographishamin. Biochem. Z - 244, 239. 1932. (E.Negelein.) Kryptohamin.Biochem.Z- 248.243.
1932. (E.Negelein.) liber Kryptohamin. Biochem. Z- 250,577.
1932. (WithW.Christian.) UbereinneuesOxydationsfermentundseinAbsorp-
tionsspektrum. Biochem. Z 2• 54, 438.
1932. (F. K ubowitz and E. H aas.) Ausbau der photochemischen Methoden zur
Untersuchung des sauerstoffubertragenden Ferments (Anwendung auf
Essigbakterien und Hefezellen.) Biochem. Z - 255, 247.
1932. (H. Schuler.) liber die Oxydation des Hamoglobineisens durch Ferri-
cyankalium und das Gleichgewicht der Reaktion. Biochem. Z - 255, 474. 1933. (C. V. Smythe.) liber die Wirkung von Blausaure auf Methylglyoxal.
Biochem. Z •257, 371.
1932. (C. V. S m y t h e .) Bildung von Brenztraubensaure aus Methylglyoxal durch
katalytische Wirkung der Blausaure. Ber. dt. chem. Ges. 65, 819.
1932. ( C . V . S m y t h e .) Oxydation von Methylglyoxal durch molekularen Sauer-
stoff bei Gegenwart von Blausaure. Ber. dt. chem. Ges. 65, 1268.
1932. (WithW.Christian.) liberdasneueOxydationsferment.JVaturwissenschaften,
20, 980.
1932. (WithW. Christian.) EinzweitessauerstofftibertragendesFermentundsein
Absorptionsspektrum. JVaturwissenschaften, 20, 688.
1932. Das sauerstofflibertragende Ferment der Atmung. Z - angewandte Chem. 45, 1. 1933. (F. K ubowitz and E. H aas.) liber das Zerstorungsspektrum der Urease.
Biochem. Z•257, 337.
1933. (WithW.Christian.) liberdasgelbeOxydationsferment.Biochem.Z- 257,
492.
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
686
[210] [211] [212] [213] [214] [215] [216] [217] [218] [219] [220] [221]
[222] [223]
[224]
1933. 1933. 1933. 1933. 1933. 1933. 1933. 1933. 1933. 1933. 1933. 1933.
1934. 1934.
1934.
Biographical Memoirs
(With W. Christian.) liber dasgelbeOxydationsferment. Biochem. Z-258,
496.
(H. Schuler.) liber das Gleichgewicht zwischen Kohlenoxydhamoglobin
und Ferricyankalium. Biochem. Z •259, 475. (C.V.Smythe andW.Gerischer.) liberdieVergarungvonHexosemono-
phosphorsaure und Glycerinaldehydphosphorsaur. Biochem. Z- 260, 414. (With W. Christian.) Sauerstoffubertragendes Ferment in Milchsaurebazil-
len. Biochem. Z- 260, 449.
(With E. N e g e l e i n .) Direkter spektroskopischer Nachweis des sauerstoffuber-
tragenden Ferments in Essigbakterien. Biochem. Z- 262, 237. (WithW.Christian.) liberdasgelbeOxydationsferment.Biochem.Z-263,
228.
(H. Gaffron.) liber den Mechanismus der Sauerstoffaktivicrung durch
belichtete Farbstoffe. Biochem. Z- 264, 251.
(W.Kempner andF.Kubowitz.) WirkungdesLichtesaufdieKohlenoxyd-
hemmung der Buttersauregarung. Biochem. Z- 265, 245.
(With E. N egelein and E. H aas.) Spektroskopischer Nachweis des sauer-
stoffiibertragenden Ferments neben Cytochrom. Biochem. Z- 266, 1.
(With W. Christian.) liber das gelbe Ferment und seine Wirkungen.
Biochem. Z- 266, 377.
(E. N e g e l e in .) liber die Extraktion eines von Bluthamin verschiedenen
Hamins aus dem Herzmuskel. Biochem. Z- 266, 412.
(E. N egelein and W. Gerischer.) Direkter spektroskopischer Nachweis des
sauerstoffiibertragenden Ferments in Azotobakter. Biochem. Z- 268, 1; also Naturwissenschaften,21, 884.
(W.Luttgens andE.Negelein.) Mikro-Zerewitinoff.Biochem.Z-269,177. (H. Gaffron.) liber die Kohlensaureassimilation der roten Schwefelbak-
terien. I. Biochem. Z- 269, 447.
(H. T h e o r e l l .) Reindarstellung (Kristallisation) des gelben Atmungs-
fermentes und die reversible Spaltung desselben. Vorlaufige Mitteilung.
Biochem. Z- 272, 155.
(WithW.Christian.) Co-Fermentproblem.Biochem.Z-274,112.
(F. K ubow itz.) liber die Hemmung der Buttersauregarung durch Kohlen-
oxyd. Biochem. Z- 274, 285.
(With E. Negelein.) Cytochrom und sauerstoffubertragendes Ferment.
Naturwissenschaften, 22, 206.
(With E. Haas.) liber eine Absorptionsbande im Gelb in Backerhefe.
Naturwissenschaften, 22, 207.
Sauerstoffiibertragende Fermente. Naturwissenschaften, 22, 441.
(H. T h e o r e l l .) Ein neuer Kataphoreseapparat fur Untersuchungszwecke.
[225] 1934. [226] 1934.
[227] 1934. [228] 1934.
[229] 1934. [230] 1935.
Biochem. Z- 275, 1.
[231] 1935. (H. T h e o r e l l .) Kataphoretische Studien iiber das Atmungs-Co-Ferment
der roten Blutzellen. Biochem. Z- 275, 11.
[232] 1935. (H. T h e o r e l l .) Bestimmung der Anzahl von sauren Gruppen des Atmungs-
Co-Ferments durch Diffusionsmessung. Biochem. Z- 275, 19.
[233] 1935. (H. T h e o r e l l .) Kataphoretische Untersuchungen l i b e r die Mischungen von
Atmungsfermenten und Substrat. Biochem. Z- 275, 30.
[234] 1935. (H. T h e o r e l l .) liber die Wirkungsgruppe des gelben Ferments. Biochem. Z-
275, 37.
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
[235] [236]
[237]
[238] [239]
[240]
[241]
[242]
[243]
[244]
[245]
[246]
[247] [248]
[249]
[250] [251] [252] [253]
[254]
[255]
[256]
[257]
[258]
[259] [260]
[261] [262]
Otto Heinrich Warburg 687 (With W. Christian.) Co-Fermentproblem. Biochem. Z- 275, 464.
(H. T h e o r e l l .) Reindarstellung der Wirkungsgruppe des gelben Fermentes. Biochem. Z- 275, 344.
(H. T h e o r e l l .) XJber Hcmmung der Reaktionsgeschwindigkeit durch Phosphat in Warburg’s und Christian’s System. Biochem. Z- 275, 416.
(H. T h e o r e l l .) Das gelbe Oxydationsferment. Biochem. Z- 278, 263.
1935. (H. T h e o r e l l .) Ein Kataphorese-apparat fur praparative Zwecke. Biochem.
Z - 278,291.
1935. (WithW.Christian andA.Griese.) DieWirkungsgruppedesCo-Ferments
aus roten Blutzellen. Biochem. Z- 279, 143.
1935. (H. T h e o r e l l .) Quantitative Bestrahlungsversuche an gelbem Ferment,
Flavinphosphorsaure und Lactoflavin. Biochem. Z- 279, 186.
1935. (W. Luttgens and W. Christian.) Ultrakohlenstoffbestimmung. Biochem. Z-
281, 310.
1935. (With W. Christian and A. Griese.) Wasserstoffiibertragendes Co-Ferment,
seine Zusammensetzung und Wirkungsweise. Biochem. • 282, 157.
1935. (E.Negelein andE.Haas.) t)berdieWirkungsweisedesZwischenferments.
Biochem. Z- 282, 206.
1935. (With W. Christian.) Zerstorung deswasserstoffubertragenden Co-Ferments
durch ultraviolettes Licht. Biochem. Z282, 221. 1935. (E. H aas.) l)ber das Absorptionsspektrum des Wassers im Ultraviolett.
Biochem. Z- 282, 224.
1935. (F. Kubowitz.) Kohlenoxyd-Ferroglutathion. Biochem. Z- 282, 277.
1936. (With W. Christian.) Pyridin, der wasserstoflfubertragende Bestandteil von
Garungsfermenten. Helv. Chim. Acta, 19, E, 79.
1936. (E. Negelein and W. Gerischer.) Verbesserte Methode zur Gewinnung des
Zwischenferments aus Hefe. Biochem. Z- 284, 289.
1936. (WithW.Christian.) Garungs-Co-Ferment.Biochem.Z-285,156.
1936. (With P. K a r r e r .) Jodmethylat des Nicotinsaureamids. Biochem. Z - 285, 297. 1936. (E. H aas.) Mikrohydrierung mit Hydrosulfit. Biochem. Z- 285, 368.
1936. (With W. Christian.) Optischer Nachweis der Hydrierung und Dehydrier-
ung des Pyridins im Garungs-Co-Ferment. Biochem. Z - 286, 81.
1936. (WithW.Christian.) PyridinalsWirkungsgruppedehydrierenderFermente.
Biochem. Z- 286, 142.
1936. (With W. Christian.) Pyridin, der wasserstoffiibertragende Bestandteil von
Garungsfermenten (Pyridin-Nucleotide.) Biochem. 287, 291.
1936. (E. Negelein.) Methode zur Gewinnung des A-Proteins der Garungs-
fermente. Biochem. Z- 287, 329.
1936. (With W. Christian.) Verbrennung von Robison-Ester durch Triphospho-
Pyridin-Nucleotid. Biochem. Z •287, 440.
1936. (E. H a a s .) Verhalten der Absorptionsspektren der Dihydro-pyridinverbin-
dungen. Biochem. Z •288, 123.
1936. Enzymes as oxygen carriers. Inst. Intern. Chim. Solvay. 5th Conference, p. 303. 1936. (With W. Christian.) Uber Nikotinsaureamid und Luminoflavin. Ber. dt.
chem. Ges. 69B, 228.
1937. (E. Negelein and H.-J. Wulff.) Kristallisation des Proteins der Acetal-
dehydreduktase. Biochem. Z 2• 89, 436.
1937. (E. H aas.) Wirkungsweise des Proteins des gelben Ferments. Biochem. •
290, 291.
1935. 1935.
1935. 1935.
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
688
[263] [264] [265] [266] [267] [268] [269]
[270]
[271]
[272]
[273]
[274]
[275]
[276]
[277]
[278] [279]
[280] [281] [282]
[283] [284]
1937. 1937. 1937. 1937. 1937. 1937. 1937.
1937.
1938.
1938.
1938.
1938.
1938.
1938.
1938.
1938. 1938.
1938. 1938. 1938.
1938. 1939.
Biographical Memoirs
(E. N e g e l e i n and H.-J. W u l f f .) Dissoziationskonstanten und Reaktionsfa- higkeit der Acetaldehydreduktase. Biochem. Z- 290, 445.
(E. H aas.) Manometrische Mikrotitration mit Ferricyanid. Biochem. Z- 291, 79.
(C. S. F r e n c h .) The quantum yield of hydrogen and carbon dioxide assimila tion in purple bacteria. J.Gen. Physiol. 20, 711. (C. S. F r e n c h .) The rate of C 0 2 assimilation by purple bacteria at various
wave lengths of light. J.Gen. Physiol. 21, 71.
(F. Kubowitz.) t)ber die chemische Zusammensetzung der Kartoffel-
oxydase. Biochem. Z- 292, 221.
(With W. C hristian.) Abbau von Robison-Ester durch Triphospho-Pyridin-
Nucleotid. Biochem. Z•292, 287.
(F. K ubowitz). Schwermetallproteid und Pyridinproteid, die Kimponenten
Blausaure—und Kohlenoxyd-empfindlicher Alkoholdehydrasen. Biochem.
Z. 293, 308.
(E. N e g e l e i n and H.-J. W u l f f .) Diphospho-pyridinproteid. Alkohol,
Acetaldehyd. Biochem. Z- 293, 351.
(With W. C hristian.) Co-Ferment der d-Aminosaure-Deaminase. Biochem.
Z. 295, 261.
(E. G. Ba l l .) Uber die Oxydation und Reduktion der drei Cytochrom-
Komponenten. Biochem. Z •295, 262.
(With W. Christian.) Co-Ferment der ^-Alanin-Oxydase. Biochem. Z- 296,
294.
(F. K u b o w i t z .) Re-Synthese der Phenoloxydase aus Protein und Kupfer.
Biochem. Z- 296, 443.
(With W. Christian and A. Griese). Alloxazin-Adenin-Dinukleotid aus
Hefe. Biochem. Z- 297, 417.
(With W. Christian.) Isolierung der prosthetischen Gruppe der d-Amino-
saureoxydase. Biochem. Z- 298, 150.
(With W. Christian.) Bemerkung uber gelbe Fermente. Biochem. Z- 298,
368.
(E. H a a s .) Isolierung eines neuen gelben Ferments. Biochem. • 298, 378.
(F. K u b o w i t z .) Spaltung und Resynthese der Polyphenoloxydase und des
Hamocyanins. Biochem. Z- 299, 32.
(E. G. Ba l l .) Xanthine oxidase: an alloxazine proteid. Science, 88, 131.
(J. N. Davidson.) Purified uricase. Nature, Lond. 141, 790.
(With W. Christian.) Koferment der d-Alanin-Dehydrase. Naturwissenschaf-
ten, 26, 201.
Chemische Konstitution von Fermenten. Ergebn. Enzymforsch. 7 , 210. (E.Negelein andH.Bromel.) Proteinderc/-Aminosaureoxydase.Biochem.Z-
300, 225.
(E.Negelein andH.Bromel.) IsolierungeinesreversiblenZwischenprodukts
der Garung. Biochem. Z- 301, 135.
(With W. Christian.) Proteinteil des kohlenhydrat-oxydierenden Ferments
der Garung. Biochem. Z• 301, 221.
(With W. Christian.) Isolierung und Kristallisation des Proteins des oxy-
[285]
[286] 1939. [287] 1939.
1939.
dierenden Garungsferments. Biochem. Z- 303, 40.
[288] 1939. (E. N egelein and H. Bromel.) R-Diphospho-glycerinsaure, ihre Isolierung
und Eigenschaften. Biochem. Z- 303, 132.
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
Otto Heinrich Warburg 689
[289] 1939. (E. N egelein and H. Bromel.) Uber die Entstehung von Glycerin bei der Garung. Biochem. Z- 303, 231.
[290] 1939. (E. G. Ball.) Xanthine oxidase: purification and properties. J. Biol. Chem. 128, 51.
[291] 1941. (F. Kubowitz and W. Luttgens.) Zusammensetzung, Spaltung und Resynthese der Carboxylase. Biochem. 307, 170.
[292] 1941. (With W. Christian.) Isolierung und Kristallisation des Garungsferments Enolase. Biochem. Z • 310, 384.
[293] 1941.
(With W. Christian.) Isolierung und Kristallisation des Garungsferments Enolase. Naturwissenschaften, 29, 589.
(With W. Christian.) Chemischer Mechanismus der Fluorid-Hemmung der Garung. Naturwissenschaften, 29, 590.
(T. Bucher and E. N egelein.) Versuche zum Quanten-Problem der Kohlensaure-Assimilation. Naturwissenschaften, 29, 591.
(E. Negelein and T. Bucher.) Photochemische Spaltung von Kohlenoxyd- Myoglobin. Naturwissenschaften 29, 672.
(With W. Christian.) Isolierung und Kristallisation des Garungsferments Enolase. Biochem. Z- 310, 384.
(T. Bucher and E. Negelein.) Photochemische Ausbeute bei der Spaltung des Kohlenoxyd-Hamoglobins. Biochem. Z- 311, 163.
(With W. Christian.) Wirkungsgruppe des Garungsferments Zymohexase. Biochem.Z. 311,209.
(F. Kubowitz and P. Ott.) Isolierung und Kristallisation eines Garungs ferments aus Tumoren. Biochem. Z- 314, 94.
(With W. Christian.) Zymohexase im Blutplasma von Tumortieren. Naturwissenschaften, 30, 731.
(With W. Christian.) Isolierung und Kristallisation des Garungsferments Zymohexase. Naturwissenschaften, 30, 731.
(F. Kubowitz and P. Ott.) Tumorferment und Muskelferment. Naturwissen schaften, 30, 732.
(T. Bucher.) Isolierung und Kristallisation eines phosphatiibertragenden Garungsferments. Naturwissenschaften, 30, 756.
(With W. Christian.) Isolierung und Kristallisation des Garungsferments Zymohexase. Biochem. Z- 314, 149.
(With W. Christian.) Garungsfermente in Blutserum von Tumor-Ratten. Biochem. Z- 314, 399.
(T. Bucher.) Optische Molekulargewichtsbestimmung an kristallisierten
Ferment- Proteinen. Angew. Chem. 56, 328.
(F. K ubowitz and P. O tt.) Isolierung von Garungsfermenten aus mensch-
lichen Muskeln. Biochem. Z• 317, 193. (With W. Luttgens.) Experiment zur Assimilation der Kohlnesaure.
Naturwissenschaften, 32, 161.
(With W. Luttgens.) Weitere Experimente zur Kohlensaureassimilation.
Naturwissenschaften, 32, 301.
(T. Bucher andJ. K aspers.) Lichtfilterfur280m/i. 33,93. (T. Bucher and J. K aspers.) Photochemische Spaltung des Kohlenoxyd-
Myoglobins durch ultraviolettes Licht. Naturwissenschaften, 33, 93. Molekulargewicht des sauerstoffubertragenden Ferments. Naturwissenschaften,
33, 94.
[294] [295] [296] [297] [298] [299] [300] [301] [302] [303] [304]
1941. 1941. 1941. 1942. 1942. 1942. 1942. 1942. 1942.
1942. 1942. 1943. 1943. 1943.
[305]
[306]
[307]
[308] 1944.
[309] [310]
[311] [312]
[313]
1944. 1944.
1946. 1946.
1946.
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
690
[314] 1946. [315] 1946. [316] 1947.
Biographical Memoirs
t)ber den Quantenbedarf der Kohlensaureassimilation. 33, 122.
(With W. Luttgens.) Photochemische Reduktion des Chinons in griinen Zellen und Granula. Biochimia, 11, 303.
(T.Bucher andJ.Kaspers.) PhotochemischeSpaltungdesKohlenoxydmyo- globins durch ultraviolette Strahlung (Wirksamkeit der durch die Protein- komponente des Pigments absorbierten Quanten). Biochim. Biophys.
1, 21.
(T. Bu c h e r .) Ober ein phosphattibertragendes Garungsferment. Biochim. Biophys. Acta, 1, 292.
(T. Bu c h e r .) Ober das Molekulargewicht der Enolase. Biochim. Biophys. Acta, 1, 467.
(T. B u c h e r .) Zum Giiltigkeitsbereich der Rayleighschen Gleichung (Moleku- largewichte aus der Lichtzerstreuung von Linearkolloiden). Biochim. Biophys. Acta, 1, 477.
Ideen zur Fermentchemie der Tumoren. Abhandlg. Dtsch. Akad. Wiss. Berlin, 3, 3.
Assimilatory quotient and photochemical yield. Amer. Botany, 35, 194. (With D. Burk, V. Schocken, S. H endricks and M. K orzenovsky.) The
maximum efficiency of photosynthesis. Science, 110, 225.
(With V. Schocken.) A manometric actinometer for the visible spectrum.
Arch. Biochem. 21, 363.
(With D. Burk, V. Schocken, M. K orzenovsky and S. B. H endricks.)
Does light inhibit the respiration of green cells?Arch. Biochem. 23, 330. (With D. Bu r k .) The maximum efficiency of photosynthesis. Arch. Biochem.
25,410.
(With D. Burk.) 1-Quanten-Mechanismus und Energie-Kreisprozess bei der
Photosynthese. Naturwissenschaften, 37, 560. (WithD.Burk,S.B.Hendricks,andV.Schocken.)Thequantumefficiency
of photosynthesis. Biochim. Biophys. Acta, 4, 335.
1-Quanten-Mechanismus der Photosynthese. Elektrochem. angew. phys.
Chem. 55, 447.
(With D. Burk.) Ein-Quanten-Reaktion und Kreisprozess der Energie bei
der Photosynthese. Z-Naturforsch. 6b, 12.
(With H. Geleick.) Ober den Gewinn im Kreisprozess der Photosynthese.
Z- Naturforsch. 6b, 134.
(With H. Geleick and K. Briese.) Ober die Aufspaltung der Photosynthese
in Lichtreaktion und Riickreaktion. Z- Naturforsch. 6b, 417.
(With H. Geleick and K. Briese.) Weitere Steigerung des Energiegewinns
im Kreisprozess der Photosynthese.
(WithH. Geleick andK. Briese.) OberdieAufspaltungderPhotosynthesein
Lichtreaktion und Riickreaktion. Z- Naturforsch. 6b, 417.
(With H.-S. G e w it z .) Cytohamin aus Herzmuskel. Z- physiol.
Chem. 288, 1.
Energetik der Photosynthese. Naturwissenschaften, 39, 337.
Der Mechanismus des Photolyse- Photosynthese-Systems der griinen Pflanzen.
Naturwissenschaften, 39, 185.
(With H. Geleick and K. Briese.) Ober die Messung der Photosynthese in
Carbonat-Bicarbonat-Gemischen. Z- Naturforsch. 7b, 141.
[317] [318] [319]
[320]
[321] [322]
1947. 1947. 1947.
1947.
1948. 1949.
[323] 1949. [324] 1949. [325] 1950. [326] 1950. [327] 1950. [328] 1951. [329] 1951. [330] 1951. [331] 1951.
[332] 1951.
[333] [334]
[335] [336]
[337]
1951. 1951.
1952. 1952.
1952.
ZNaturfor
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
[338] [339] [340]
[341] [342]
[343]
[344] [345] [346] [347] [348] [349]
[350] [351] [352] [353] [354] [355]
[356]
[357] [358] [359]
[360]
1952. 1952. 1952.
1952. 1953.
1953.
1953. 1953. 1953. 1953. 1954. 1954.
1954. 1954. 1954. 1954. 1954. 1954.
1954.
1954. 1954. 1955.
1955.
Otto Heinrich Warburg 691
(With E. H iepler.) Versuche mit Ascites-Tumorzellen. 7b, 193.
t)ber den Einfluss der Wellenlange auf die Chinonreduktion in griinen Grana. Z-Naturforsch. 7b, 443.
(WithD.Burk andA.L.Schade.) Extensionsofphotosyntheticexperimenta tion. In: Symposia of the Society for Experimental Biology V. Carbon dioxide
fixation and photosynthesis, Cambridge University Press, p. 306.
(H. T iedemann.) Gber den Stoffwechsel des Ascites-Tumors der Maus.
Z-ges. exptl. Med. 119, 272.
(WithK.Damaschke,F.Todt andD. Burk.) Anelectrochemicaldemonstra
tion of the energy cycle and maximum quantum yield in photosynthesis.
Biochim. Biophys. Acta, 12, 347.
(With G. Krippahl, W. Schroder and W. Buchholz.) Messung des
Quantenbedarfs der Photosynthese fur sehr diinne Zellsuspensionen.
Biochim. Biophys. Acta, 12, 356.
(With G. K rippahl, W. Buchholz and W. Schroder.) Weiterentwicklung
der Methoden zur Messung der Photosynthese. Naturforsch. 8b, 675. Gber die Wirkungsgruppen der oxydierenden und reduzierenden Fermente.
Naturwissenschaften, 40, 493.
Gber das Verhalten von Aszites-Tumorzellen zu Sauerstoff von hoheren
Drucken. Arch. Geschwulstorsch, 6, 7.
(With H.-S. G e w it z .) Cyto-Deutero-Porphyrin. Z- physiol.
Chem. 292, 174.
(With K. Gawehn and G. Lange.) Gber das Verhalten von Ascites-Krebs-
zellen zu Zymohexase. Z - Naturforsch. 9b, 109.
(With G. K rippahl, W. Schroder, W. Buchholz and E. T heel.) Gber die
Wirkung sehr schwachen blaugriinen Lichts auf den Quantenbedarf der
Photosynthese. Z•Naturforsch. 9b, 164.
(With G. Krippahl.) Messung der Lichtabsorption in Chlorella mit der
Ulbrichtschen Kugel. Z •Naturforsch. 9b, 181. (With K. G a w e h n .) Isolierung der Hefezymohexase und ihre Kristallisation
als Quecksilbersalz. Z- Naturforsch. 9b, 206.
Gber die Beriicksichtigung der Retention der Kohlensaure bei Messungen
der Photosynthese in Kulturldsungen. Z- Naturforsch. 9b, 302.
(With H. Klotzsch and K. Gawehn.) Gber die Oxydationsreaktion der
Garung. Z- Naturforsch. 9b, 391.
Gber Phosphorylierung durch Dehydrierung. Supplement to Z- Naturforsch.
9b, 391.
(With G. K rippahl, and W. Schroder.) Katalytische Wirkung des blau-
griinen Lichts auf den Energieumsatz bei der Photosynthese. Z- Natur forsch. 9b, 667.
(With G. Krippahl, W. Schroder and W. Buchholz.) Sauerstoff- Kapazitat der Chlorella. Z- Naturforsch. 9b, 769.
(With G. K r ip p a h l .) Gber Photosynthese-Fermente. Angew. Chem. 66, 493. Krebsforschung. Naturwissenschaften, 41, 485.
(G. K r i p p a h l .) Carotinoid als Wirkungsgruppe eines Photosynthese-
Ferments. Ann. Acad. Scient. Fennicae. 60, 69.
(With G. Krippahl.) Photochemische Wasserzersetzung durch lebende
Chlorella. Z- Naturforsch. 10b, 301.
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
692
Biographical Memoirs
[361] 1955. (With G. K rippahl and W. Buchholz.) Wirkung von Vanadium auf die Photosynthese. Z- Naturforsch. 10b, 422.
[362]
[363]
[364]
[365]
[366]
[367]
[368] [369]
[370]
[371]
[372]
[373]
[374]
[375]
[376]
[377]
[378] [379] [380]
[381]
[382] [383]
[384] [385] [387]
1955. (With H.-S. Gewitz and W. Volker.) Fiber Gewinnung und Abbau des Cytohamins. Z- Naturforsch. 10b, 541.
1955.
1955.
1955.
1955.
1955.
1955. 1955.
1955. 1955. 1956. 1956. 1956. 1956. 1956. 1956.
1956. 1956. 1956.
1956.
1956. 1956.
1956. 1956. 1957.
(With G. K rippahl and W. Schroder.) Wirkungsspektrum eines Photo- synthese-Ferments. Z- Naturforsch. 10b, 631.
(With W. Schroder.) Versuche liber die Sauerstoff-Kapazitat der Chlorella. Z- Naturforsch. 10b, 639.
(H.-S. Gewitz and W. Volker.) Fiber die Synthese einiger Deuteropor- phyrine. Hoppe-SeylePsZ- physiol. Chem. 302, 119.
(WithG.Krippahl.) Activityspectrumofa photosyntheticenzyme.Pubbl. Staz. Zool.Napoli. 27, 1.
Fiber die Messung des Energieumsatzes bei der Photosynthese mit dem Grossflachen-Bolometer. Biochim. Biophys. Acta, 18, 163.
t)ber die Entstehung der Krebszellen. Naturwissenschaften, 42, 401. Photodissoziation und induzierte Atmung, die Fundamental-Reaktionen der
Photosynthese. Naturwissenschaften, 42, 449.
I. Fiber die Entstehung der Krebszellen. II. Experimente liber Photosynthese.
Mitteilungen aus der Max-Planck-Gesellschaft 4, 166.
t)ber die Entstehung der Krebszellen. In Krebsforschung und Krebsbekampfung
( Sonderbande zur trSahlentherapie,34), p. 3.
(With G. Krippahl.) Fiber die funktionelle Kohlensaure der Chlorella.
Z- Naturforsch. lib, 718.
(With G. Krippahl.) Fiber die C02-Kapazitat der Chlorella und den
chemischen Mechanismus der C02—Assimilation. Naturforsch. lib, 52. (With G. Krippahl.) Fiber die funktionelle Carboxylgruppe des Chloro
phylls. Z- Naturforsch. lib, 179.
(With W. Schroder and H.-W. Gattung.) Ziichtung der Chlorella mit
fluktuierender Lichtintensitat. Z- Naturforsch. lib, 654.
(With K. Gawehn and A.-W. Geissler.) Stoffwechsel von embryonalen
Zellen und von Krebszellen. Z- Naturforsch. l i b , 657.
(With G. Krippahl.) Fiber die funktionelle Kohlensaure der Chlorella.
Z•Naturforsch. 11b, 718.
On the origin of cancer cells. Science, 123, 309.
On respiratory impairment in cancer cells. Science, 124,269.
(With G. Krippahl andW. Schroder.) FiberdenchemischenMechanismus
der Kohlensaureassiniilation. Naturwissenschaften, 43, 237.
(G. K rippahl and W. Schroder.) Uber den chemischen Mechanismus der
Kohlensaure-Assimilation. Angew. Chem. 68, 418.
Uber die Entstehung der Krebszellen. Oncologia, 9 (2), 75.
Krebsforschung. From ‘ Ausder Deutschen Forschung der letzen
Ernst Telschow zum 65. Geburtstag gewidmet. (Edited by B. Rajewsky and
G. Schreiber.) Stuttgart: Georg Thieme Verlag.
(D. Burk and A. L. Schade.) On respiratory impairment in cancer cells.
Science, 124, 270.
Chemischer Mechanismus der C 0 2—Assimilation und die Theorie von
Willstatter-Stoll. Festschrift Arthur Stoll, Basel: Sandoz A.G. p. 705. Stoffwechsel von embryonalen Zellen in Gewebekutluren. Biochem. Biophys.
Acta, 25, 429.
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
[388] 1957.
[389] 1957.
[390] 1957.
[391] 1957.
[392] 1957.
[393] 1957.
[394] 1957.
[395] 1957.
[396] 1957.
[397] 1957.
[398] 1957.
[399] 1957.
[400] 1957.
[401] 1957.
[402] 1958.
[403] 1958.
[404] 1958.
[405] 1958.
[406] 1958.
[407] 1958.
[408] 1958.
[409] 1958.
[410] 1958.
Otto Heinrich Warburg 693
(With K. Gawehn andA.-W. Geissler.) ManometriederKorperzellenunter physiologischen Bedingungen. Z- Natur12b, 115.
(With K. Gawehn and A.-W. Geissler.) Stochiometrische Versuche mit dem oxydierenden Garungsferment. Naturforsch. 12b, 47.
(H.Klotzsch andG.Krippahl.) DberdieFunktionderGlutaminsaurein Chlorella. Z- Naturforsch. 12b, 266.
(With K. Gawehn and A.-W. Geissler.) Dber die Wirkung von Wasser- stoffperoxyd auf Krebszellen und auf embryonale Zellen. Z- Naturforsch. 12b, 393.
(With H. K lotzsch and G. K rippahl.) Dber dasVerhalten einiger Amino- sauren in Chlorella bei Zusatz von markierter Kohlensaure. Z - , 12b 481.
(With H. K lotzsch and G. K rippahl.) Glutaminsaure in Chlorella. Z- Naturforsch. 12b, 622.
(WithW.Schroder.) QuantenbedarfderPhotosynthese.Z-Naturforsch.12b, 716.
(With H.-S. Gewitz and W. Volker.) D(—•)Milchsaure in Chlorella. Z-Naturforsch. 12b, 722.
(With W. Schroder, G. K rippahl and H. K lotszch.) Photosynthese. Angew. Chem. 69, 627.
(D. Bu r k .) Dber die Begriindung einer Chemotherapie des Krebses auf der Grundlage einer primaren Hemmung der Glucosephosphorylierung. Klin. Wschr. 35, 1102.
(With H. K lotzsch and G. K rippahl.) Glutaminsaure-Decarboxylase in Chlorella. Naturwissenschaften, 44, 235.
(WithG.Krippahl.) AusbaudermanometrischenMethoden.JustusLiebigs Annalen Chem. 604, 94.
Paul Ehrlich 1954-1915. In Die Grossen Deutschen, Bd. 4, p. 186. Propylaen Verlag, Berlin.
Atmungshemmung und Karzinogenese. Experientia, 13, 125.
(With K. Gawehn and A.-W. Geissler.) Dber die Entstehung des Krebs-
stoffwechsels in der Gewebekultur. Z- Naturforsch. 13b, 61.
(With G. K r ip p a h l .) Beweis der Notwendigkeit der Glutaminsaure fur die
Photosynthese. Z-Naturforsch. 13b, 63.
(With G. K r ip p a h l .) Sauerstoff-Halbwertdrucke der Photosynthese und
Atmung. Z- Naturforsch. 13b, 66.
(With G. K r ip p a h l .) Weiterentwicklung der manometrischen Methoden.
Z- Naturforsch. 13b, 434.
(WithG.Krippahl,H.-S.Gewitz andW.Volker.) Carotinoid-Oxygenase
in Chlorella. Z- Naturforsch. 13b, 437.
(With G. Krippahl.) Hill-Reaktionen. Z- Naturforsch. 13b, 509.
(With K. Gawehn and A.-W. Geissler.) Stoffwechsel der weissen Blutkor-
perchen. Z •Naturforsch. 13b, 515.
(With K. Gawehn and A.-W. Geissler.) Katalasegehalt, Garung und
Atmung in der Zellkultur nach Dulbecco-Vogt. Z - Naturforsch. 13b, 588. (With W. Schroder, H.-S. Gewitz and W. Volker.) Manometrisches Rontgenstrahlen-Aktinometer und iiber die Wirkung der Rdntgenstrahlen
auf die Garung von Krebszellen. Z •Naturforsch. 13b, 591.
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
694
[411] [412] [413] [414] [415]
[416] [417]
1958. 1958. 1958. 1958. 1958.
1958. 1959.
Biographical Memoirs
(WithG.Krippahl, H.-S.Gewitz andW.Volker.) tlberdenchemischen MechanismusderPhotosynthese. Z-14b 712.
(WithG.Krippahl andW.Schroder.) UberdenchemischenMechanismus
der Kohlensaureassimilation. Naturwissensch43, 237.
(With W. Schroder, H. Gewitz and W. Volker.) tjber die selektive WirkungderRontgenstrahlenaufKrebszellen.Naturwissenschaften,45, 192. (With W. Schroder, G. K rippahl and H. K lotzsch.) Photosynthesis.
Angew. Chem. 669, 627. (WithK.Gawehn,A.-W.Geissler,W.Schroder,H.-S. Gewitz andW.
V o l k e r .) Partielle Anaerobiose und Strahlenempfindlichkeit der Krebs
zellen. Arch. Biochem. Biophys. 78, 573.
Photosynthesis. Science, 128, 68.
(With K. Gawehn, A.-W. Geissler, W. Schroder, H. Gewitz and W.
V o l k e r .) Partielle Anaerobiose der Krebszellen und Wirkung der Ront
genstrahlenaufKrebszellen.Naturwissenschaften,46, 25. (WithG.Krippahl,H.Gewitz andW.Volker.)Uberdenchemischen
Mechanismus der Photosynthese. Angew. Chem. 71, 633.
(With G. Krippahl.) Weiterentwicklung der manometrischen Methoden.
Z- Naturforsch. 14b, 561.
(With D. K a y s e r .) Wirkung von Kohlenoxyd a u f Atmung und Photo
synthese in griinen Keimblattern. Z - Naturforsch. 1 4 b , 563. (WithG.Krippahl,H.-S.Gewitz andW.Volker.)Uberdenchemischen
Mechanismus der Photosynthese. Z >Naturforsch. (With G. K r i p p a h l .) Further development of manometric methods. J . Nat.
CancerInst. 24, 51.
(WithW.Schroder andH.-W.Gattung.) OberdieWirkungvonRontgen
strahlen auf Hamoglobin (Mit einer Bemerkung uber die Strahlenem pfindlichkeit von Gewebeschnitten.) Z - Naturforsch. 1 5 b , 163.
[418]1959.
[419] [420] [421] [422] [423]
[424]
[425] [426]
[427]
1959. 1959. 1959. 1960. 1960.
1960.
1960. 1960.
1960.
(With G. K r i p p a h l .) Neubestimmung des synthese mit der Kompensations-methode.
Quantenbedarfs der Photo Naturforsch. 1 5 b , 190.
Z - Naturforsch. 1 5 b , 197. (Carbonatgemische.) Z•Naturforsch. 15b, 364.
(With G. K r i p p a h l .) Notwendigkeit der Kohlensaure fur die Chinon- und Ferricyanid-Reaktionen in griinen Grana. Z - Naturforsch. 1 5 b , 367.
(WithG. K r i p p a h l .) Glykolsaurebildung in
(With G. K r i p p a h l .) Weiterentwicklung der manometrischen Methoden.
[428] 1960. (With G. K rippahl and H.-W. Gattung.) 1-Gefass-Methode zur Messung des Quantenbedarfs der Photosynthese. Z - Naturforsch. 1 5 b , 370.
[429]1960. (WithK.Gawehn,andA.-W.Geissler.)Umwandlungdesembryonalen Stoffwechsels in Krebsstoffwechsel. Z- Naturforsch. 15b, 378.
[430] 1960. (H.-S. G e w i t z and W. V o l k e r .) Weiterentwicklung der manometrischen Methoden (Herstellung definierter Blausaure-Konzentrationen). Z - Naturforsch. 15b, 625.
[431] 1960. (With G. K r i p p a h l .) Weiterentwicklung der manometrischen Methoden (Wechsel aerober und anaerober Bedingungen.) Z - Naturforsch. 1 5 b , 786.
[432] 1960. (With G. K r i p p a h l .) Uber den Photolyten der Photosynthese. Z - Naturforsch. 1 5 b , 788.
[433] 1960. (WithK. Gawehn andA.-W. Geissler.) Weiterentwicklungderzellphysiolo- gischen Methoden (Verbindung von Manometrie und optischer Milch- saurebestimmung). Hoppe-Seyler’s Z-hpChem. 320, 277.
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
[434]
1960.
1961. 1961. 1961. 1961. 1962.
1962. 1962.
1962. 1962. 1962. 1962. 1962.
1962. 1962.
1963.
1963. 1963. 1963.
1963. 1963. 1963. 1963.
Otto Heinrich Warburg 695
(With K. G a w e h n , A.-W. G exssler and S. L o r e n z .) Uber die Umwandlung des embryonalen StofFwechsels in Krebsstoffwechsel. Z-
physiol. Chem. 321, 252.
Geschichte des Max-Planck-Instituts fur Zellphysiologie. Jahrbuch
Planck-Gesellschaft, II, 816.
(With A.-W. Geissler and S. Lorenz.) C02-Drucke iiber Bicarbonat-
Carbonatgemischen. Z-Naturforsch. 16b, 283.
(H.-S. G e w i t z and W. V o l k e r .) 3-Hydroxytyramin, ein biologischer
Oxydationskatalysator der Photosynthese. Z- Naturforsch. 16b, 559.
tJber die fakultative Anaerobiose der Krebszellen und ihre Anwendung auf
die Chemotherapie. Munch. Med. Wschr. 103, 2504.
(WithG. Krippahl,A.-W.Geissler andS.Lorenz.)Weiterentwicklungder
manometrischen Methoden (Sauerstoffabsorption durch Chromchloriir).
Z- Naturforsch. 17b, 17.
(With G. K rippahl.) MessungvonAutoxydationen. Z- Naturforsch. 17b, 281. (WithG. Krippahl,A.-W.Geissler andS.Lorenz.) Sauerstoffabsorption
durch Chromchlorur. Z- Naturforsch. 17b, 281.
(With G. K r ip p a h l .) Ziichtung von Chlorella mit der Xenon-Hochdruck-
lampe. Z- Naturforsch. 17b, 631.
(With G. Krippahl.) Weiterentwicklung der manometrischen 1-Gefass-
methoden. Z •Naturforsch. 17b, 631.
(D. K ayser.) Uber die Messung des Sauerstoffdruckes, bei dem Sporen von
Clostridium butyricum auskeimen. Z- Naturforsch. 17b, 658.
(WithA.-W. Geissler andS.Lorenz). AtmungundWachstumvonKrebs
zellen bei niedrigen Sauerstoffdrucken. Z- Naturforsch. 17b, 758.
(With K. Gawehn, A.-W. Geissler and S. Lorenz.) Uber den Einfluss des Sauerstoffdrucks auf die Umwandlung des embryonalen Stoffwechsels in
Krebsstoffwechsel. Z•Naturforsch. 17b, 772.
On phosphorylation in light. J. Gen. Physiol. 45, (Pt. 2), 17.
In La Photosynthese. Colloques Internationaux du Centre National de la Recherche
Scientifique, 119, 221.
(B. Vennesland, H. W. Gattung and E. Birkicht.) Fluorometric measure
ment of the photoreduction of flavin by illuminated chloroplasts. Biochim.
Biophys. Acta, 66, 285.
(D. K a y s e r .) Liber die Hemmung des Wachstums von Bacterium lactis
aerogenes durch Rontgenstrahlen. Z- Naturforsch. 18b, 171.
(With G. K rippahl.) Optische Bestimmung von Wasserstoffperoxyd mit
Vanadinsaure. Z~Naturforsch. 18b, 340.
(H.-S. Gewitz and W. Volker.) Hemmung der Photosynthese durch
Kohlenoxyd und Aufhebung der Kohlenoxydhemmung durch Licht.
Z- Naturforsch. 18b, 649.
(With K. Gawehn, A.-W. Geissler and S. Lorenz.) Liber Abtotung von
Krebszellen durch Rontgenstrahlen. Z- Naturforsch. 18b, 654.
(D. K ayser.) Liber den Mechanismus der Abtotung von Ascites-Krebszellen
durch Clostridium butyricum, Z- Naturforsch. 18b, 748.
(With G. K rippahl, K. J etschmann and A. Lehmann.) Chemie der Photo
synthese. Z- Naturforsch. 18b, 837.
(With P. O stendorf.) Quantenbedarf der Photosynthese in Blattern. Z-
Naturforsch. 18b, 933.
[435] [436] [437] [438] [439]
[440] [442]
[443] [444] [445] [446] [447]
[448] [449]
[450]
[451] [452] [453]
[454] [455] [456] [457]
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
696 Biographical Memoirs
[458] 1963. Weiterentwicklung der zellphysiologischen Methoden. Klin. Chem. 1, 33. [459] 1963. (With K. Gawehn, A.-W. Geissler and S. Lorenz.) Dber Heilung von Mause-Ascites-Krebs durch D-und L-Glycerinaldehyd. Z - Klin.Chem. 1, 175. [460] 1963. (With G. Krippahl.) Ober den Einfluss des Kohlensauredrucks auf den
[461]
[462]
[463]
[465]
[466]
[467] [468]
[469] [470] [471] [472]
[473]
[474] [475] [476]
[477]
[478] [479]
[480] [481]
[482]
1963.
1964.
1964.
1964.
1964.
1964. 1964.
1964. 1964. 1965. 1965.
1965.
1965. 1965. 1966.
1966.
1966. 1966.
1966. 1966.
1967.
Quantenbedarf der Photosynthese. Acta Chem. Scand. 17, Suppl. 1,1.
(With G. Krippahl.) Die chemischen Gleichungen der Photosynthese.
Hoppe-Seyler s Z-physiol. Chem. 332, 225.
(With G. K rippahl and E. Birkicht.) Quantenchemie der Photosynthese,
automatisch geschrieben. Biochem. Z - 340, 1.
(WithG.Krippahl.) fiberdasVerhaltenvonAsparaginsaureundAlaninin
Chlorella, untersucht mit optischen Testen. Biochem. Z- 340, 471.
(With K. Gawehn, A.-W. Geissler, D. Kayser and S. Lorenz.) The
mechanism of biological X-rays action. Naturwissenschaften, 51, 373. Berichtigung. Bemerkung zu dem Nachruf von W. Kroebel aufJames Franck.
Naturwissenschaften, 51, 550.
Prefatory-chapter. Ann. Rev. Biochem. 33, 1. (WithG.Krippahl,K.Jetschmann andA.Lehmann.) Chimiedelaphoto
synthese. Bull. Soc. Chim. Biol. 46, 9.
(D. K a y s e r .) tJber die Wirkung von Chinonen auf Ascites-Krebszellen in vivo
and in vitro. Z - Naturforsch. 19b, 258.
(With E. Birkicht, G. K rippahl andA. Lehmann.) Newmethodsin measur
ing 1-quantumreactionofphotosynthesis.Ber.BunsenGes.Phys.Chem.68,767. (With K. Gawehn, A.-W. Geissler, D. Kayser and S. Lorenz.) Experi-
mente zur Anaerobiose der Krebszellen. Klin. Wschr. 43, 289.
(With G. K rippahl and K. J etschmann.) Widerlegung der Photolyse des Wassers und Beweis der Photolyse der Kohlensaure nach Versuchen mit
lebender Chlorella und den Hill-Reagentien Nitrat und K3Fe(CN).
Z- Naturforsch. 20b, 993.
(WithA.-W.GeisslerandS.Lorenz.) MessungderSauerstoffdruckebeim
Umschlag des embryonalen Stoftwechsels in Krebs-Stoffwechsel. Z-
Naturforsch, 20b, 1070.
Gerhard Domagk, Dtsch. A.ledWsch. 90, 1484.
On the mechanism of photosynthesis. Agrochimica, 9, 105.
(With G. Krippahl.) Uber das Einsteinsche Gesetz und die Funktion des
roten Ferments bei der Photosynthese. Biochem. 344, 103.
Vber die letzte Ursache und die entfernten Ursachen des Krebses. Wurzburg: Verlag
K. Triltsch.
Causes of cancer. Colloq. Ges. Physiol. Chem. 17, 1.
(D. K a y s e r .) t)ber die Wirkung von Rontgenstrahlen auf das Wachstum
von Lactobacillus Delbriickii und iiber die Rolle von Metallspuren beim
Zustandekommen der biologischen Rontgenstrahlenwirkungen. Z- Natur forsch. 21b, 167.
(With A.-W. Geissler and S. Lorenz.) Irreversible Erzeugung von Krebs- stoffwechsel in embryonalen Mausezellen. Z- Naturforsch. 21b, 707.
Oxygen, the creator of differentiation, in Current aspects of bioenergenetics (Edited by N. O. Kaplan and E. P. Kennedy), p. 103. London, New York: Academic Press.
(With A.-W. Geissler and S. Lorenz.) Bemerkung liber die Tryptophan- Oxygenase. Hoppe-SeylePs Z- physiol. Chem. 348, 899.
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
[483] 1967. [484] 1967.
[485] 1967.
[486] 1967.
[487] 1967.
[488] 1967. [489] 1967.
[490] 1967. [491] 1968.
[492] 1968. [493] 1968.
[494] 1969. [495] 1969. [496] 1969.
[497] 1969.
[498] 1969. [499] 1970. [500] 1970. [501] 1970.
[502]1970. [503] 1970.
Otto Heinrich Warburg 697
Neue manometrische Zweigefassmethode. 1677.
(With A.-W. Geissler and S. Lorenz.) Wirkung von Riboflavin und von S-Aminolavulinsaure auf wachsende Krebszellen in vitro.
Z- physiol. Chem. 348, 1683.
(WithA.-W.Geissler andS.Lorenz.) tJberWachstumvonKrebszellenin Medien, deren Glucose durch Galaktose ersetzt ist. Hoppe-Seyler’s Z- physiol. Chem. 348, 1686.
(With E. Birkicht and R. Stevens.) Quantenbedarfder Bilanz von Licht- reaktion und Ruckreaktion bei der Photosynthese, automatisch geschrie- ben. Biochem. Z •346, 407.
(With G. K r ip p a h l .) Photolyt und Chlorophyll in Chlorella. Biochem. Z- 346, 418.
(WithG.Krippahl.) PhotolytundManganinChlorella.Biochem.Z-346,429. (With G. Krippahl and A. Lehmann.) tjber Photolyt und Phosphat in
Chlorella. Biochem. Z- 346, 434.
Gedenkwortefur GerhardDomagk. Heidelberg: Verlag Lambert Schneider. (With G. K rippahl and A. Lehmann.) Metaphosphate and aerobic C02in
Chlorella. FEBS Lett. 1, 171.
(WithA.-W. Geissler andS.Lorenz.) WirkungvonRiboflavinundLumino-
flavin auf wachsende Krebszellen. Z- Chem. 6, 467.
(With G. Krippahl and A. Lehmann.) Dialysierte ein neues
Versuchsmaterial zur Untersuchung der Photosynthese. 23b, 1076.
The prime cause and prevention o f cancer. English edition by D. Burk. Wurzburg: Verlag K. Triltsch.
(With G. K rippahl and A. Lehmann.) Dber die Chlorophyll-Katalyse der Photosynthese. Z •Naturforsch. 24b, 1355.
(With G. K rippahl and A. Lehmann.) liber das mit Kohlensaure verbun- dene und das freie Chlorophyll bei der Photosynthese. Z- Naturforsch. 24b, 1583.
(With G. K rippahl and A. Lehmann.) Chlorophyll catalysis and Einstein’s law of photochemical equivalence in photosynthesis. Amer. J. Botany, 56, 961.
(With G. K rippahl and A. Lehmann.) Chlorophyll catalysis and Einstein’s photochemical law in photosynthesis. FEBS Lett. 3, 221.
(With E. Birkicht and G. Pahlke). Anderung desChlorophyllspektrums bei Bildung und Zerfall des Photolyten. Z- Naturforsch. 25b, 112.
(With A.-W. Geissler and S. Lorenz). Entstehung von Krebsstoffwechsel durch Vitamin-Bj-Mangel (Thiaminmangel). Z- Naturforsch. 25b, 332.
(With A.-W. Geissler and S. Lorenz.) liber die Erzeugung von normalem Stoffwechsel aus Krebsstoffwechsel durch Zusatz von Vitamin-Bs. Z- Naturforsch. 25b, 559.
(WithE.Birkicht,G.KrippahlandG.Pahlke.)Changesofthechloro phyll spectrum in living Chlorella, if the photolyte is split by light. FEBS Lett. 8, 247.
Biologische Manometrie. In Methoden der enzymatischen Analyse, (Ed. H.-U. Bergmeyer), p. 208. Weinheim: Verlag Chemie.
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
698
[504] 1926.
[505] 1928.
Biographical Memoirs Books
Vber den Stoffwechsel der
English by F. Dickens under the title The metabolism of tumours. London: Constable & Co. 1930. (A collection of original papers published previously in various journals, prefaced by a chapter on methodology).
Vber die katalytischen Wirkungen der lebendigen Substanz. Berlin: Verlag Springer. (A collection of papers published previously in various journals, prefaced by a chapter summarising the work as a whole).
[506] 1946. •Schwermetalle als Wirkungsgruppe von Fermenten. Berlin: Verlag Saenger. Translated by A. Lawson into English under the title Heavy metals and enzyme action. Oxford: Clarendon Press, (1949). (A review of Warburg’s work on the subject and a discussion of related work by other authors).
[507] 1949. Wasserstoffiibertragende Fermente. Editio Cantor GMBH, Freiburg i. Br. (A collection of papers published previously, mainly in Biochem. prefaced by two extensive review articles).
[508] 1962. Weiterentwicklung der zellphysiologischen Methoden, angewandt auf Photo- synthese und Wirkungsweise der Rontgenstrahlen.
New methods o f cell physiology, applied to cancer, photosynthesis, and mechanism of X-ray action. George Thieme Verlag, Stuttgart and Interscience, New York. (This book contains an introduction summarising the work in both German and English. In addition it contains a biographical sketch of Otto Warburg by D. Burk and 94 separate papers, most of which have been published elsewhere, with the following exceptions:
(a) (With G. K rippahl.) Manometrische Bestimmung der Ascorbin- saure, p. 541.
(b) (With A. Lehmann and H. W. Gattung.) t)ber den Quanten- bedarf der Photosynthese in Blattern, p. 542.
(c) (With G. Krippahl.) Weitentwicklung der manometrischen Methoden, p. 555.
(d) (T. Terranova and K. Gawehn.) Stoffwechsel der Zellen des trypsinisierten Knochenmarks, p. 557.
(e) (With G. K r i p p a h l .) Ortho- und meta-Phenanthrolin in Chlorella, p. 560.
(f) (With K. Gawehn and T. T erranova.) Weitere Versuche fiber die Umwandling des embryonalen Stoffwechsel, p. 562.
(g) (With A.-W. Geissler and S. Lorenz.) Neue Methode zur Bestim mung der Kohlensauredrucke iiber Bicarbonat-Carbonatgemi- schen, p. 578.
(h) (With A.-W. Geissler and S. Lorenz.) Quantenausbeute der Photosynthese als Funktion des Kohlensauredrucks, p. 582.
(i) (With A.-W. Geissler and S. Lorenz.) Weitere Versuche iiber die
Wirkung von Rontgenstrahlen auf den Stoffwechsel der Krebs-
zellen, p. 585.
(j) (WithD.Kayser.) tjberdieWirkungderRontgenstrahlenaufden
Stoffwechsel von Milchsaurebakterien, p. 586.
(k) (With G. Krippahl, H. Gewitz and W. Volker.) Uber Sauer-
stoffiibertragung durch belichtetes Chlorophyll, p. 595.
.neromuTBerlin: Verlag Springer. Transla
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
Otto Heinrich Warburg 699
(l) (With G. K r ip p a h l .) Wasserstoffperoxyd in Chlorella, p. 606.
(m) (WithG.Krippahl andK.Jetschmann.) ZerfallundWiederaufbau
der Glutaminsaure bei Chlorella, p. 609.
(n) (With K. J etschmann.) Hemmung der Phosphorylierung durch
Phenanthrolin in lebender Chlorella, p. 612.
(o) (With K. J etschmann.) Wirkung der Blausaure aufdie Bildung des
Photolyten der Photosynthese, p. 615.
(p) (WithK.Gawehn.)tjberdieMessungderAtmungvonKrebszel-
len mit einer 1-Gefassmethode, p. 618.
(q) (With D. K ayser.) t)ber Wachstumshemmung durch Rontgen-
strahlen, p. 620.
(r) tJber Phosphorylierung im Licht, p. 622.
(s) Beweis, dass der Photolyt der Photosynthese eine Kohlensaurever-
bindung ist, p. 625.
(t) t)ber die fakultative Anaerobiose der Krebszellen und ihre Anwendung auf die Chemotherapie, p. 627.
(u) TJber die Klassifizierung der Krebszellen auf der Basis der Anaero
biose in vivo, p. 631.
(v) t)ber Phosphorylierung durch Dehydrierung, p. 633.
Downloaded from https://royalsocietypublishing.org/ on 04 November 2024
https://royalsocietypublishing.org/doi/pdf/10.1098/rsbm.1972.0023