They are all together in this criminal act for mass murder based on the „Volksgesundheit“ public health Example under the Nazis Rule!
From the Clintons, to Bush, to Obama, to Trump, to Biden, to Fauci & the NIH to the FDA, to most of the US Government and the CIA, all members of the WEF, NSA and NASA, from Epstein to all the Maxwells, to all members of the Edge Foundation, from the Gates Fondation & Microsoft to the WHO, from the Rockefellers and the Club of Rome, to Antonio Guteres and the UN, from the John Hopkins University to the University of North Carolina Chappel Hill and Ralph Baric to the EcoHealth Alliance and Peter Daszak, from Stanford, to Havard, to the MIT, to Oxford, from the Charitee to Christian Drosten, Kristian Anderson, Neil Ferguson to the Wellcome Trust, from Facebook to Mark Zuckerberg & Dustin Moskowitz to Twitter and Elon Musk, from Peter Thiel to Palantir to Alex Karp to all members of the Paypal Mafia, from Amazon to all the Media and also most of the so called alternative Media they are all guilty! And this is just the tip of the iceberg.
So let me explain those plans by lawmaking acts that proof that this bollwerk is build to harm, it’s rotten to the core and should by definition legally handled as a conspiring act and Covid was only a little step on the way …to a fascist system of permanent surveillance, social credit scores and the license to kill, all disguised under the umbrella of “public health”, “doing good” and „ for humankind“.
Point of View
No Person or artificial algorithm or even all money and all the smartest people in the world together have the capacity or the potential to create a vaccine in 100 days that is safe and effective!
Nobody and nothing can do this!
Because the timeframe is the Clou of safe and effective that allows the professional and carefully acting scientist to study and proof the material of his outcomes.
To Take Time means you take care and that costs a lot of money, clearly!
So you can’t change the direction and put the same money in a shorter time periode to have the same Result of Quality!
Thats is physically Impossible and in the real world of good manufacturing practice Undisputed!
Quantity and Quality are firmly connected to each other in an inesitable relationship. Like the Moon to the Earth or Quantum mechanics. That means if you change a composition on the one side you always change the direction at the other side!
When we have reached the point and forget logical thinking and lost touch with reality?
https://julimination.wordpress.com/2024/05/14/this-is-not-a-vaccine-by-definition/
Public Health Emergency Declaration
The Secretary of the Department of Health and Human Services (HHS) may, under section 319 of the Public Health Service (PHS) Act, determine that: a) a disease or disorder presents a public health emergency (PHE); or b) that a public health emergency, including significant outbreaks of infectious disease or bioterrorist attacks, otherwise exists.
Duration and Notification: The declaration lasts for the duration of the emergency or 90 days, but may be extended by the Secretary. Congress must be notified of the declaration within 48 hours, and relevant agencies, including the Department of Homeland Security, Department of Justice, and Federal Bureau of Investigation, must be kept informed.
Prior to issuing the declaration, the Secretary should consult with public health officials as necessary.
Following a section 319 declaration, the Secretary can:
Take appropriate actions in response to the emergency consistent with other authorities, including: making grants; entering into contracts; and conducting and supporting investigations into the cause, treatment, or prevention of the disease or disorder. Upon request of the recipient of any such award and subject to corresponding reductions in payments, the Secretary may also provide supplies, equipment, and services, and detail employees of the Department to the recipient to aid the recipient in carrying out the award.
Access “no-year” funds appropriated to the Public Health Emergency Fund to rapidly respond to immediate needs resulting from the PHE, or to rapidly respond to a potential PHE when the Secretary determines that there is a significant potential for a PHE. The Fund may be used to facilitate coordination among federal, state, local tribal, and territorial entities and public and private health care entities affected by the PHE; to make grants, provide for awards, enter into contracts and conduct investigations including further supporting the Public Health Emergency Preparedness, Hospital Preparedness and Regional Health Care Emergency Preparedness awards; facilitate and accelerate advanced research and development of medical countermeasures; strengthen biosurveillance and laboratory capacity; support initial emergency operations related to preparation and deployment of National Disaster Medical System teams; and carry out other activities determined applicable and appropriate by the Secretary. The Secretary must report to Congress 90 days after the end of the fiscal year about any funds spent from the Public Health Emergency Fund, the emergency for which funds were spent, and activities undertaken with respect to the emergency. Public Health Emergency Funds supplement, but do not supplant, other Federal, State, and local funds provided for public health grants, awards, contracts, and investigations.
Enable the Centers for Disease Prevention and Control Director to access the Infectious Diseases Rapid Response Reserve Fund (when funds are so appropriated) to prevent, prepare for, or respond to an infectious disease emergency, either when the Secretary has declared a public health emergency or when the Secretary determines that the emergency has significant potential to imminently occur and potential on occurrence, to affect national security or the health and security of US citizens, domestically, or internationally.
Grant extensions or waive sanctions relating to submission of data or reports required under laws administered by the Secretary, when the Secretary determines that, wholly or partially as a result of a public health emergency, individuals or public or private entities are unable to comply with deadlines for such data or reports. The Secretary must notify Congress and publish a Federal Register notice before or promptly after granting an extension or waiver.
Waive or modify certain Medicare, Medicaid, Children’s Health Insurance Program (CHIP), and Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule requirements. Under section 1135 of the Social Security Act (SSA), the Secretary may waive or modify certain requirements as necessary to ensure to the maximum extent feasible that, in an emergency area during an emergency period, sufficient health care items and services are available to meet the needs of individuals Medicare, Medicaid, and CHIP, and that providers of such services in good faith who are unable to comply with certain statutory requirements are nonetheless reimbursed and exempted from sanctions for noncompliance, absent fraud, or abuse. There must also be a Presidential declaration of an emergency or disaster in order to exercise this authority.
Adjust Medicare reimbursement for certain Part B drugs. Most Medicare Part B drugs are paid on the basis of the manufacturer’s average sales price (ASP), which manufacturers are required to report quarterly. The ASP-based payment allowance is updated prospectively each quarter, using the data manufacturers report. The statutory scheme results in a two-quarter lag between the date of the reported sale and the date that sale’s price is factored into the Medicare reimbursement rate. In the case of a public health emergency in which there is a documented inability to access drugs and biologicals and a concomitant increase in the price of a drug or biological that is not reflected in the manufacturer’s ASP for one or more quarters, the Secretary may use the wholesale acquisition cost or other reasonable measure of drug or biological price instead of the manufacturer’s ASP. The substituted price or measure may be used until the price of the drug or biological has stabilized and is substantially reflected in the manufacturer’s ASP. As of April 2013, CMS has not formally interpreted, nor exercised, this authority. However, in the event of a public health emergency in which this authority were triggered, the price change could be implemented without rulemaking. (See Section 1847A(c)(5)(C) of the Social Security Act, which states that notwithstanding any other provision of law, the Secretary may implement any of the provisions of Section 1847A by program instruction or otherwise.)
Make temporary (up to one year or the duration of the emergency) appointments of personnel to positions that directly respond to the public health emergency when the urgency of filling positions prohibits examining applicants through the competitive process.
Use funds from the fiscal year 2019 appropriation to HHS that are available for salaries and expenses of HHS employees to pay travel and related expenses of an employee or family member when the employee is assigned to duty in the U.S. or in a U.S. territory during a period and in a location that are the subject of a public health emergency declaration, and travel is necessary to obtain medical care for an illness, injury, or medical condition that cannot be adequately addressed in that location at that time.
Enable the Secretary of Defense, in consultation with the Secretary, to deploy military trauma care providers providing care at high-acuity trauma centers pursuant to grants awarded under section 1291 of the PHS Act.
Waive certain Ryan White HIV/AIDS program requirements (section 2683 of the PHS Act). Under this authority, up to five percent of the funds available under each of the Parts A and B supplemental pools may be shifted to ensure access to care during a public health emergency declared by the Secretary under section 319 of the PHS Act or an emergency or disaster declared by the President under the Stafford Act or the National Emergencies Act in the geographic area where the emergency, major disaster, or public health emergency exists. In addition, the Secretary may waive such requirements of title XXVI of the PHS Act to improve the health and safety of those receiving care under this title and the general public. This waiver authority is limited to the time period for which the emergency, major disaster, or public health emergency declaration exists.
Modify practice of telemedicine. The Ryan Haight Online Pharmacy Consumer Protection Act and implementing regulations allow the Secretary, with concurrence of the DEA Administrator, to designate patients, patient locations, and use of controlled substances during a public health emergency declared by the Secretary.
On a State by State basis, as the circumstances of the emergency reasonably require and for the period of the emergency, grant an extension or waive application deadlines or compliance with any other requirement of certain SAMHSA grants. Such grants include those authorized under sections 521, 1911, or 1921 of the PHS Act. This authority also applies to allotments authorized under Public Law 99-319.
Allow State and local governments to access the General Services Administration (GSA) Federal supply schedule when using federal grant funds. GSA policy allows state, local, and tribal government grantees to use federal supply schedules to respond to public health emergencies declared by the Secretary. Pursuant to 48 U.S.C. § 1469e as amended, Puerto Rico, Guam, American Samoa, the Commonwealth of the Northern Mariana Islands, and the U.S. Virgin Islands may always buy off of the GSA schedules regardless of whether there is a declared PHE.
Temporarily reassign state and local personnel. The Secretary may authorize a requesting Governor of a state or tribal organization to temporarily reassign state and local public health department or agency personnel funded in whole or in part through programs authorized under the PHS Act to immediately address a public health emergency in the state or Indian tribe during the period of the emergency.
Limit liability of health care professionals who are members of the Medical Reserve Corps or professionals included in the Emergency System for Advance Registration of Volunteer Health Professionals responding to a PHE in the initial 90 days so that such professionals shall be subject only to the State liability laws in which the professional has been deployed to the PHE and only to the extent permitted under the laws of the State in which care is provided.
Determine a waiver of Paperwork Reduction Act (PRA) requirements is necessary. Section 319(f), recently added by the 21st Century Cures Act, allows the Secretary to determine that the circumstances of a PHE or a disease or disorder, including a novel and emerging public health threat that is significantly likely to become a PHE, necessitate a waiver from PRA requirements. If the Secretary makes such a determination, then PRA requirements for voluntary collection of information do not apply during the immediate investigation of and response to the PHE during the period of the PHE or the time period necessary to determine if a disease or disorder, including a novel and emerging public health threat, will become a PHE.
Waive certain requirements of the Drug Supply Chain Security Act (DSCSA). A public health emergency is considered an “emergency medical reason” under the DSCSA, 21 U.S.C. 360eee. Thus, upon the Secretary’s declaration of a public health emergency, certain activities are automatically excluded through the time period of the declaration. Notably, product distribution for such emergency medical reasons is excluded from the DSCSA definitions of “transaction” and “wholesale distribution.” Therefore, the DSCSA requirements related to product tracing and wholesale distribution do not apply to trading partner activities that address the public health emergency declaration.
Enable the Department of Labor (DOL) to issue dislocated worker program grants for disaster relief employment pursuant to 29 U.S.C. § 3225. A Federal agency emergency or disaster declaration or a Stafford Act declaration triggers an opportunity for eligible entities to apply for disaster relief employment National Dislocated Worker Grants. In order for a Federal agency declaration to trigger this authority, the chief official of a Federal agency with authority for or jurisdiction over the Federal response must declare or otherwise recognize an emergency or disaster situation of national significance that could result in a potentially large loss of employment.
Public Health Emergency
Legal Authorities
Legal Authorities Overview
Public Health Service Act
The PHS Act forms the foundation of HHS’ legal authority for responding to public health emergencies. Among other things, it authorizes the HHS Secretary to lead all Federal public health and medical response to public health emergencies and incidents covered by the National Response Framework; to direct the U.S. PHS and other components of the Department to respond to a public health emergency; to declare a public health emergency (PHE) and take such actions as may be appropriate to respond to the PHE consistent with existing authorities; to assist states in meeting health emergencies; to control communicable diseases; to maintain the Strategic National Stockpile; to provide for the operation of the National Disaster Medical System; to establish and maintain a Medical Reserve Corps; and to potentially provide targeted immunity for covered countermeasures to manufacturers, distributors, certain classes of people involved in the administration of a program to deliver covered treatments to patients, and their employees. The PHS Act was amended by the Pandemic and All-Hazards Preparedness Act of 2006 (PAHPA) by the Pandemic and All-Hazards Preparedness Reauthorization Act (PAHPRA) of 2013, by the 21st Century Cures Act of 2016, and most recently by the Pandemic and All Hazards Preparedness and Advancing Innovation Act of 2019 (PAHPAIA), which have broad implications for the Department’s preparedness and response activities.
Social Security Act
The Social Security Act authorizes Medicare, Medicaid, the Children’s Health Insurance Program (CHIP), and social services programs of the Department. It authorizes the Secretary, among other things, to temporarily modify or waive certain Medicare, Medicaid, CHIP, and HIPAA requirements when the Secretary has declared a public health emergency and the President has declared an emergency or a major disaster under the Stafford Act or a national emergency under the National Emergencies Act.
Federal Food, Drug and Cosmetic Act
The Federal Food, Drug, and Cosmetic Act is the foundation for FDA’s authority and responsibility to protect and promote the public health by, among other things, ensuring the safety and effectiveness of human and veterinary drugs, biological products, and medical devices; and ensuring the safety and security of our nation’s food supply. When certain conditions have occurred, it authorizes the Secretary to declare an emergency justifying emergency use authorization (EUA) of unapproved drugs, devices, or biological products, or emergency use authorization of approved drugs, devices, or biological products for an unapproved use. For more information about EUA, please see Food and Drug Administration, Emergency Use Authorization.
Robert T. Stafford Disaster Relief and Emergency Assistance Act
At the request of the Governor of an affected State, or a Chief Executive of an affected Indian Tribe, the President may declare a major disaster or emergency if an event is beyond the combined response capabilities of the State, Tribal, and jurisdictional governments. Among other things, this declaration allows Federal assistance to be mobilized and directed in support of State, Tribal, and jurisdictional response efforts. Under the Stafford Act (42 USC Chapter 68), the President can also declare an emergency without a Gubernatorial request if primary responsibility for response rests with the Federal Government because the emergency involves a subject area for which the United States exercises exclusive responsibility and authority. In addition, in the absence of a specific request, the President may provide accelerated Federal assistance and Federal support where necessary to save lives, prevent human suffering, or mitigate severe damage, and notify the State of that activity.
Legal Authority of the Secretary
Legal Authority without Declaration of a Public Health Emergency
Legal Authority with Declaration of a Public Health Emergency
Legal Authority When the President Declares a Major Disaster or an Emergency
Emergency Authority When the President and the HHS Secretary Issue a Declaration
Disclaimer: This webpage is intended to provide general information about the HHS Secretary’s legal authorities to prepare for and respond to public health and medical emergencies and is not intended to provide specific legal advice or guidance. This information does not provide an exhaustive list of the HHS Secretary’s legal authorities to prepare for and respond to public health and medical emergencies. Individuals should always seek the advice of an attorney with any questions they may have regarding a legal matter.
The HHS Secretary has legal authority to take action to prepare for and respond to public health and medical emergencies under several statutes, primarily including the Public Health Service (PHS) Act; Federal Food, Drug and Cosmetic Act; and the Social Security Act. Various other legal authorities may also authorize the HHS Secretary to respond to public health and medical emergencies.
In general, the legal authority of the HHS Secretary for each of the following circumstances is:
1. Legal Authority without Declaration of a Public Health Emergency
Even without the HHS Secretary declaring a public health emergency under section 319 of the PHS Act, he/she has broad legal authorities to provide assistance to states and local entities and to conduct studies. Some examples of actions that the HHS Secretary may take, if appropriate, include:
Developing and taking necessary steps to implement a plan to assist states and localities to control epidemics and to meet other health emergencies or problems
Enabling the Centers for Disease Control and Prevention Director to access the Infectious Diseases Rapid Response Reserve Fund (when funds are so appropriated) to prevent, prepare for, or respond to an infectious disease emergency when the emergency has significant potential to imminently occur and to affect national security or the health and security of US citizens, domestically or internationally
Access “no-year” funds appropriated to the Public Health Emergency Fund (when funds are so appropriated) to immediately respond when the Secretary determines that there is a significant potential for a PHE
Assisting and promoting research and studies into the causes, diagnosis, treatment, control, and prevention of diseases
Establishing isolation and quarantine
Maintaining the Strategic National Stockpile (SNS)
Activating the U.S. Public Health Service (USPHS) Commissioned Corps and the National Disaster Medical System (NDMS), and deploying select members of the Medical Reserve Corps (MRC)
Maintaining safety of food, drugs, biological products, and medical devices
Providing temporary assistance to needy families and responding to needs of “at-risk” individuals
Providing, through a separate declaration, immunity from liability for activities related to supply, distribution, and use of medical countermeasures to chemical, biological, radiological, nuclear, and pandemic and epidemic threats
Providing, through a separate declaration, that FDA may authorize medical products intended to prevent, diagnose, or treat a disease or condition involving a specified biological, chemical, radiological, or nuclear agent or agents, or intended to prevent, diagnose, or treat a serious or life-threatening disease or condition caused by such a product described for emergency use
Waive certain requirements for drugs covered by risk evaluation and mitigation strategies
Permit the dispensing of medical products intended to prevent, diagnose, or treat a disease or condition involving a biological, chemical, radiological, or nuclear agent or agents, or intended to prevent, diagnose, or treat a serious or life-threatening disease or condition caused by such a product without a prescription for the duration of an emergency situation
Exempting (for 30 days, subject to one 30-day renewal) a person from select agents requirements as necessary to provide for timely participation of the person in a response to a domestic or foreign public health emergency that involves the select agent or toxin
Waive requirements of the Paperwork Reduction Act for voluntary collection of information when necessary to prepare and respond to a disease or disorder that is significantly likely to become a public health emergency
2. Legal Authority with Declaration of a Public Health Emergency
The HHS Secretary is authorized to take the following actions when a Public Health Emergency is declared.
Consistent with regular authorities*:
Make grants to State and local agencies
Provide awards for expenses
Enter into contracts
Conduct and support investigations into the cause, treatment, or prevention of the specific disease or disorder
Provide supplies, equipment, and services, and detail employees of the Department to the recipients of such awards upon request of the recipient and subject to corresponding reductions in payments
Access funds appropriated to the Public Health Emergency Fund (when funds are so appropriated) to immediately respond to the PHE
Enable the Centers for Disease Control and Prevention Director to access the Infectious Diseases Rapid Response Reserve Fund (when funds are so appropriated) to prevent, prepare for, or respond to an infectious disease emergency
Grant extensions or waive sanctions related to deadlines for submitting data or reports required under laws administered by the Secretary
Make temporary appointments of personnel (up to one year or the duration of the emergency) to respond to the public health emergency*
Pay travel and related expenses of an HHS employee (or family member) assigned to duty in an area in of the U.S. subject to a public health emergency declaration to obtain medical care for an illness, injury, or medical condition that cannot be adequately addressed in the area at that time
Enable the Secretary of Defense, in consultation with the Secretary, to deploy military trauma care teams (if established under grants awarded by the Secretary to eligible high-acuity trauma centers).
Modify practice of telemedicine
Allow State and local governments to access the General Services Administration Federal supply schedule when using federal grant funds
Allow temporary reassignment of State and local personnel during a public health emergency
Limit liability of volunteer health care professionals during the first 90 days of the PHE to the laws of the State to which the professional has been deployed to respond to the PHE and in which care is provided.
Adjust Medicare reimbursement for certain Part B Drugs
Waive certain Ryan White HIV/AIDS program requirements
Grant an extension or waive application deadlines or compliance with any other requirement of certain SAMHSA grants
Waive requirements of the Paperwork Reduction Act for voluntary collection of information when necessary to prepare for and respond to the public health emergency
Waive certain requirements of the Drug Supply Chain Security Act (DSCSA)
A public health emergency declaration may also be the basis for the Department of Labor to award dislocated worker program grants for disaster relief.
A declaration of a public health emergency terminates after 90 days or when the Secretary declares that the emergency no longer exists, unless renewed by the Secretary.
3. Legal Authority When the President Declares a Major Disaster or an Emergency
In addition to his regular authorities, the HHS Secretary may be authorized or directed to take other actions when the President declares a major disaster or an emergency under the Robert T. Stafford Act or an emergency under the National Emergencies Act.
The President may declare a major disaster under the Stafford Act when a natural catastrophe (i.e., hurricane, tornado, earthquake, etc.) or, regardless of cause, any fire, flood, or explosion causes damage that in the determination of the President is of sufficient severity and magnitude to warrant major disaster assistance to supplement the efforts and available resources of states, local governments, and disaster relief organizations in alleviating the damage, loss, hardship, or suffering caused thereby. The Stafford Act also authorizes the President to declare an emergency when, in the determination of the President, federal assistance is needed to supplement state and local efforts and capabilities to save lives and to protect property and public health and safety, or to lessen or avert the threat of a catastrophe.
The Stafford Act authorizes a multitude of Federal agency actions in response to a major disaster or emergency, such as the following:
Issuance of warnings
Mobilization of emergency support teams
Authorization of federal use of state and local government services and facilities
Hiring temporary personnel
Limiting liability of volunteer health care professionals to the laws of the State to which the provider has been deployed to respond to the disaster or emergency and in which care is provided
Distributing food, medicine, and supplies
Coordinating with private sector disaster relief organizations
Overseeing mass feeding
Coordinating hazard mitigation
Use, donation, or lending of federal equipment, supplies, facilities, or personnel to state and local governments*
Provide crisis counseling (only in response to a major disaster)
The President may also declare a National Emergency under the National Emergencies Act, specifying which statutory authorities available for use in an emergency will be exercised.
4. Emergency Authority When the President and the HHS Secretary Issue a Declaration
When the President declares a major disaster or an emergency and the HHS Secretary declares a public health emergency, the Secretary is authorized to take certain actions in addition to his regular authorities. For example, under section 1135 of the Social Security Act, he may temporarily waive certain Medicare, Medicaid, and Children’s Health Insurance Program (CHIP) requirements to ensure that sufficient health care items and services are available to meet the needs of individuals enrolled in Social Security Act programs in the emergency area and time periods. Examples of these 1135 waivers or modifications include:
Certain conditions of participation, certification requirements, program participation, or similar requirements for individual health care providers or types of health care providers
Preapproval requirements
State licenses for physicians and other healthcare professionals (this waiver is for purposes of Medicare, Medicaid, and CHIP reimbursement only – the state determines whether a non-federal provider is authorized to provide services in the state without state licensure)
Emergency Medical Treatment and Labor Act (EMTALA) sanctions for direction or reallocation of an individual to another location to receive medical screening pursuant to an appropriate state emergency preparedness plan or a state pandemic preparedness plan for the transfer of an individual who has not been stabilized if the transfer is necessitated by the circumstances of the declared federal public health emergency. A waiver of EMTALA requirements is effective only if actions under the waiver do not discriminate on the basis of a patient’s source of payment or ability to pay.
Stark self-referral sanctions
Performance deadlines and timetables may be adjusted (but not waived).
Limitations on payment for healthcare items and services to permit Medicare Advantage Plan enrollees to use out of network providers in an emergency situation
In addition, the Secretary may waive Health Insurance Portability and Accountability Act (HIPAA) Privacy rule sanctions and penalties relating to the following:
Obtaining a patient’s consent to speak with family members or friends
Honoring a patient’s request to opt out of the facility directory
Distributing a note of privacy practices
Honoring the patient’s right to request privacy restrictions or confidential communications
The waiver of HIPAA requirements is effective only if actions under the waiver do not discriminate on the basis of a patient’s source of payment or ability to pay.
These waivers under section 1135 of the Social Security Act typically end with the termination of the emergency period, or 60 days from the date the waiver or modification is first published unless the Secretary of HHS extends the waiver by notice for additional periods of up to 60 days. Waivers for EMTALA (for emergencies that do not involve a pandemic disease) and HIPAA requirements are limited to a 72-hour period beginning upon implementation of a hospital disaster protocol. Waiver of EMTALA requirements for emergencies that involve a pandemic disease last until the termination of the pandemic related emergency. The waiver for licensure applies only to federal requirements and does not automatically apply to state requirements for licensure or conditions of participation.
* The HHS Secretary has other authorities that may permit him to take similar actions even in absence of a public health emergency declaration.
Emergency Use Authorization
FDA may issue an EUA for an unapproved drug, biological product, or device; or for an unapproved use of a drug, biological product or device following a Declaration by the Secretary of Health and Human Services that the circumstances justify such authorization based on one of the following:
A determination by the Secretary of Homeland Security that there is an actual or significant potential for a domestic emergency involving a heightened risk of attack with a biological, chemical, radiological, or nuclear agent(s);
A determination by the Secretary of Homeland Security of a material threat of a chemical, biological, radiological or nuclear agent sufficient to affect national security or the health and security of United States citizens living abroad;
A determination by the Secretary
of Defense that there is a military emergency, or a significant potential for a military emergency, involving a heightened risk to United States
military forces, including personnel operating under the authority of title 10 or title 50, of attack with (i) a biological, chemical, radiological, or nuclear agent or agents; or (ii) an agent or agents that may cause, or are otherwise associated with, an imminently life-threatening and specific risk to United States
military forces; or
A determination by the Health and Human Services Secretary that there is a public health emergency or a significant potential for a public health emergency that affects, or has significant potential to affect, national security or the health and security of U.S. citizens living abroad and involves a biological, chemical, radiological, or nuclear agent(s) or disease or condition that may be attributable to such agent(s).
FDA may then issue an EUA for a product if FDA finds that:
the agent specified in the declaration of emergency can cause a serious or life-threatening disease or condition;
based on the totality of scientific evidence available, it is reasonable to believe that the product may be effective in diagnosing, treating, or preventing the serious or life-threatening disease or condition, or a serious or life-threatening disease or condition caused by a product authorized approved, cleared, or licensed by FDA for diagnosing, treating, or preventing the disease or condition;
the known and potential benefits of the product outweigh the known and potential risks of the product when used to diagnose, prevent, or treat the serious or life-threatening disease or condition, taking into consideration the material threat posed by the agent or agents identified in a declaration by the DHS Secretary, if applicable;
there is no adequate, approved, and available alternative to the product for diagnosing, preventing, or treating such serious or life-threatening disease or condition; and
in the case of a determination by the Secretary of Defense regarding risk to U.S. military forces from agents other than chemical, biological, radiological and nuclear agents, that the request for emergency use is made by the Secretary of Defense.
FDA will, in issuing the EUA, impose conditions on the emergency use that is authorized. For more information on the emergency use authority, please see Emergency Preparedness and Response: Emergency Use Authorization.
Pandemic and All Hazards Preparedness Act
In December 2006, Congress passed and the President signed the Pandemic and All-Hazards Preparedness Act (PAHPA), Public Law No. 109-417, which has broad implications for the Department of Health and Human Service’s (HHS) preparedness and response activities. Among other things, the Act amended the Public Health Service Act to established within the Department a new Assistant Secretary for Preparedness and Response (ASPR); provided new authorities for a number of programs, including the advanced development and acquisitions of medical countermeasures; and called for the establishment of a quadrennial National Health Security Strategy.
The purpose of the Pandemic and All-Hazards Preparedness Act is “to improve the Nation’s public health and medical preparedness and response capabilities for emergencies, whether deliberate, accidental, or natural.”
Major Program Areas
Biomedical Advanced Research and Development Authority (BARDA) and Medical Countermeasures;
Emergency Support Function (ESF) #8: Public Health and Medical Response: Domestic Programs;
Emergency Support Function (ESF) #8: Public Health and Medical Response: International Programs;
Grants;
At-Risk Individuals;
Situational Awareness: Surveillance, Credentialing, and Telehealth; and
Education and Training
Pandemic and All-Hazards Preparedness Reauthorization Act
In March 2013 Congress passed and the President signed the Pandemic and All-Hazards Preparedness Reauthorization Act (PAHPRA), Public Law No. 113-5. The 2013 law builds on work the U.S. Department of Health and Human Services has undertaken to advance national health security. These include authorizing funding for public health and medical preparedness programs, such as the Hospital Preparedness Program and the Public Health Emergency Preparedness Cooperative Agreement, amending the Public Health Service Act to grant state health departments greatly needed flexibility in dedicating staff resources to meeting critical community needs in a disaster, authorizing funding through 2018 for buying medical countermeasures under the Project BioShield Act, and increasing the flexibility of BioShield to support advanced research and development of potential medical countermeasures. PAHPRA also enhances the authority of the U.S. Food and Drug Administration to support rapid responses to public health emergencies.
The purpose of the Pandemic and All-Hazards Preparedness Reauthorization Act is “to reauthorize certain programs under the Public Health Service Act and the Federal Food, Drug, and Cosmetic Act with respect to public health security and all-hazards preparedness.”
Major Program Areas
National Advisory Committee on Children and Disasters
Temporary reassignment of State and local personnel during a public health emergency
Improving State and local public health security
Hospital preparedness and medical surge capacity
Enhancing situational awareness and biosurveillance
Enhancing medical countermeasure review
Accelerating medical countermeasure advanced research and development
and All Hazards Preparedness Act (PAHPA) > Pandemic and All-Hazards Preparedness and Advancing Innovation Act
Pandemic and All-Hazards Preparedness and Advancing Innovation Act
On June 24, 2019 Congress passed and the President signed the Pandemic and All-Hazards Preparedness and Advancing Innovation Act (PAHPAIA), Public Law No. 116-22. The 2019 law amends the Public Health Service Act to build on work the U.S. Department of Health and Human Services has undertaken to advance national health security. Amendments include enhancing the authorities of the Secretary, Assistant Secretary for Preparedness and Response, and the Director of the Centers for Disease Control and Prevention to prepare for and respond to public health emergencies. PAHPAIA authorizes new public health and medical preparedness programs for regional health care preparedness and military and civilian partnerships; reauthorizes funding and enhances authorities for the Hospital Preparedness Program, the Public Health Emergency Preparedness Cooperative Agreement program and other public health and medical preparedness programs.
PAHPAIA also authorizes uses for the Public Health Emergency Fund when the Secretary declares a public health emergency or determines that there is a significant potential for a public health emergency and authorizes advance funding for buying medical countermeasures under the Project BioShield Act and to funding to support advanced research and development of potential medical countermeasures.
PAHPAIA also amends the Federal Food, Drug & Cosmetic Act to enhance the authority of the U.S. Food and Drug Administration to support rapid responses to public health emergencies.
Major Program Areas
Regional Health Care Emergency Preparedness and Response Systems
Military and Civilian partnership for trauma readiness
Strengthening and assessing the emergency response workforce
National Advisory Committee on Seniors and Disasters
National Advisory Committee on Individuals with Disabilities and Disasters
Improving State and local public health security
Hospital preparedness and medical surge capacity
Enhancing situational awareness and biosurveillance
Accelerating medical countermeasure advanced research and development and supporting procurement
Public Readiness and Emergency Preparedness Act
The Public Readiness and Emergency Preparedness Act (PREP Act) authorizes the Secretary of the Department of Health and Human Services (Secretary) to issue a declaration (PREP Act declaration) that provides immunity from liability (except for willful misconduct) for claims of loss caused, arising out of, relating to, or resulting from administration or use of countermeasures to diseases, threats and conditions determined by the Secretary to constitute a present, or credible risk of a future public health emergency to entities and individuals involved in the development, manufacture, testing, distribution, administration, and use of such countermeasures. A PREP Act declaration is specifically for the purpose of providing immunity from liability, and is different from, and not dependent on, other emergency declarations.
Current Declarations
Ebola Disease Vaccines - Amendment (effective December 1, 2018)
Ebola Disease Therapeutics - Amendment (effective December 1, 2018)
Pandemic Influenza Medical Countermeasures (amended effective January 1, 2016)
Anthrax Medical Countermeasures (amended effective January 1, 2016)
Acute Radiation Syndrome Medical Countermeasures (amended effective January 1, 2016)
Botulinum Toxin Medical Countermeasures (amended effective January 1, 2016)
Smallpox Medical Countermeasures (amended effective January 1, 2016)
Control of Dual-threat Agents: The Vaccines for Peace Programme ???
Erhard Geissler and John P. Woodall
ISBN 0-19-829172-8
1994
Oxford University Press
The Vaccines for Peace (VFP) programme is designed to counter reservations about the military misuse of research into vaccines. It focuses on vaccines developed to combat those pathogens and toxins which can be used as weapon agents. In this book a range of experts from fields as diverse as biotechnology, vaccinology, international law, and diplomacy discuss the VFP programme.
Contents
Part I. Introduction
1. Introduction (Erhard Geissler and John P. Woodall)
2. The Vaccines for Peace programme: Executive summary (Erhard Geissler and John P. Woodall)
3. Arms control, health care and technology transfer under the Vaccines for Peace programme (Erhard Geissler)
Part II. Strengthening the Biological Weapons Convention
4. The missing link in implementation of the BWC: The war against pathogens (Félix C. Calderón)
5. Vaccines for Peace: A political scientist's critique (Oliver Thränert)
6. Control and co-operation in biological defence research: national programmes and international accountability (Nicholas A. Sims)
7. Verification of the BWC (Tibor Tóth, Erhard Geissler and Thomas Stock)
8. The conversion of biological warfare research and development facilities to peaceful uses (Milton Leitenberg)
Part III. Vaccine R&D and production
9. Viral vaccines: Their evolution and current trends (Florian Horaud)
10. Human viral vaccines (Nizar Ajjan, Pierre Saliou and Louis Valette)
11. Viral vaccine research, development and production in the former Soviet Union: A brief review of the situation (Sergey G. Drozdov)
12. The Scientific and Production Association Vector: The current situation (Sergey V. Netesov)
13. Vaccines against dual-threat agents: Regulation and quality, issues and constraints (Jack Melling)
Part IV. Public health and biological defence
14. Vaccines for biological defence: Defence considerations (Graham S. Pearson)
15. Molecular aspects of the activity and design of antiviral agents (Peter Langen)
16. Vaccines for public health: Can Vaccines for Peace help in the war against disease? (Stephen S. Morse)
17. The Global Epidemiological Surveillance System and Vaccines for Peace: Complementary initiatives in public health and weapon control (Mark L. Wheelis)
Part V. International programmes for prevention of disease
18. The WHO global smallpox eradication programme: Vaccine supply and variola virus stocks (Frank Fenner)
19. Options for the development and transfer of vaccine technology (Devaguptapu Subrahmanyam)
20. Funding and managing international programmes in biosciences and health care (John P. Woodall)
Part VI. Final discussion
21. From BVI to VFP: Towards a system of global biological security (Erhard Geissler, Félix C. Calderón and John P. Woodall)
Part VII. Annexes
Annexe A. The 1972 Biological Weapons Convention
Annexe B. Final Declaration of the Third Review Conference of the BWC
Annexe C. The Biesenthal Vaccine Initiative (Vaccines for Peace) Biesenthal Consensus
Annexe D. The Biesenthal Vaccine Initiative Mission Statement
Annexe E. Mission Statement of the Federation of American Scientists (FAS) Project on the Global Control of Emerging Infectious Diseases
Annexe F. US House of Representatives Resolution H.R. 5241
About the editors
Dr Erhard Geissler is Professor of Genetics and Head of the Bioethical Research Group, Max Delbrück Centre for Molecular Medicine, Berlin-Buch. A biologist by training, he has done research in the fields of biophysics, microbial genetics, and tumour virology. He has also published extensively on the subjects of biological weapons, the social impact of genetics and other biosciences, and the responsibility of scientists, and has convened several symposia on these questions. He edited Biological and Toxin Weapons Today (1986) and Strengthening the Biological Weapons Convention by Confidence-Building Measures (1990), No 10 in the SIPRI Chemical & Biological Warfare Studies.
Dr John P. Woodhall, an epidemiologist and virologist, was leader of the World Health Organization's observer delegations to the Third Review Conference of the Biological Weapons Convention (BWC) and the four meetings of the Ad Hoc Group of Governmental Experts to Identify and Examine Potential Verification Measures from a Scientific and Technical Standpoint in 1992-93. He is also on the Steering Committees of the Biesenthal Vaccine Initiative and of the Global Program for Monitoring Emerging Diseases, another initiative connected with strengthening the BWC. He has been published in earlier volumes of this series and in other journals on the epidemiology of agents relevant to the BWC.
SIPRI Chemical & Biological Warfare Studies is a series of studies intended primarily for specialists in the field of CBW arms control and for people engaged in other areas of international relations or security affairs whose work could benefit from a deeper understanding of particular CBW matters.
Ordering details
This book can be ordered from all good bookshops and online booksellers or directly from OUP
OUP in the UK: http://www.oup.com/uk/catalogue/?ci=9780198291725 (paperback)
OUP in the USA: http://www.us.oup.com/us/catalog/general/?view=usa&ci=9780198291725(paperback)
Historical Timeline
The pre-history of EUA
1950
The first event to foreshadow the major themes surrounding FDA’s EUA power was the Thalidomide tragedy of the mid-20th century. In the 1950s, a new drug called Thalidomide was put into circulation in West Germany and other countries as a treatment for morning sickness. The next decade would reveal that the drug resulted in severe birth defects, with known cases numbering in the tens of thousands. The drug’s introduction to market is remembered as one of the worst public health disasters in recent times. It underscored the importance of strict standards of clinical review in approving new food and drug products, and remains a key reference point for FDA regulators, emphasizing the importance of the agency’s extensive and thorough formal approval process.
Operation Whitecoat
Operation Whitecoat was a biodefensemedical research program carried out by the United States Army at Fort Detrick, Marylandbetween 1954 and 1973. The program pursued medical research using volunteer enlisted personnel who were eventually nicknamed „Whitecoats“. These volunteers, all conscientious objectors, including many members of the Seventh-day Adventist Church, were informed of the purpose and goals of each project before providing consent to participate in any project. The stated purpose of the research was to defend troops and civilians against biological weapons. A Consent Statement (1955) for one of the Operation Whitecoat experiments at Fort Detrick
Although the program was discontinued in 1973, human use research for biodefense purposes is still conducted at the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID) at Fort Detrick and at other government and civilian research institutes. However, these post-Whitecoat studies are often human use challenge studies, in which a person is inoculated with a known pathogen to determine how effective an investigational treatment will be.
Results
Many of the vaccines that protect against biowarfare agents were first tested on humans in Operation Whitecoat.[4]
According to USAMRIID, the Whitecoat operation contributed to vaccines approved by the U.S. Food and Drug Administration(FDA) for yellow fever and hepatitis, and investigational drugs for Q fever, Venezuelan equine encephalitis, Rift Valley fever, and tularemia. USAMRIID also states that Operation Whitecoat helped develop biological safety equipment, including hooded safety cabinets, decontamination procedures, fermentors, incubators, centrifuges, and particle sizers.[5]
Discontinuation
Operation Whitecoat came to an end in 1973 when the draft for the U.S. military ended and thus no more conscientious objectors were to be conscripted.
1976
Fast forward to 1976, when reports of cases involving a new strain of influenza A (the same family of flu viruses that caused the flu pandemic of 1918) prompted fears of a possible “swine flu” pandemic. President Gerald Ford pushed for a first-ever national vaccination program — shortly before starting his reelection campaign. After millions had been vaccinated, the public was alarmed by reports that the vaccine might be causing Guillain-Barré syndrome. And ultimately, a pandemic never materialized.
In their post-mortem study, Richard Neustadt and Harvey Fineberg described the swine flu episode as a policymaking disaster; yet they also expressed concern that the American public and policymakers would wrongly oversimplify the event in their memory as a case of government overreaction and overstepping, and that they would thus over-learn the dangers of responding too swiftly to fears of a pandemic. (Some would argue that this concern did not bear out, as nothing was learned at all.)
1980
Next, the AIDS crisis gave rise to an early precursor to EUA authority. In the late 1980s, public health experts suggested that an investigational drug called DDI might prove useful for AIDS patients unable to tolerate other medications. Many objected that DDI lacked formal approval and was not guaranteed to be safe and effective; others countered that the risks of breaking protocol by issuing a drug lacking formal approval paled in comparison to the number of lives that could be saved. Impatient with FDA regulators’ conservative approach, Dr. Anthony Fauci, Director of the National Institute of Allergy and Infectious Diseases, proposed a new “parallel track” system to administer DDI to eligible patients while continuing to study the drug. Other measures for circumventing FDA’s formal approval process already existed at the time, but this proposal attracted the attention of President George H.W. Bush, whose support encouraged FDA to adopt the process and administer DDI to those in need.
1986
Congress passed the National Childhood Vaccine Injury Act ("Vaccine Act")
The federal government plays a leading role in vaccination by funding vaccine administration and by integrating the immunization efforts of the public and private sectors on national, state and local levels. Congress repeatedly reaffirms this role of the federal government in order to ensure that the United States maintains a consistent national policy on childhood vaccination. In 1986, Congress passed the National Childhood Vaccine Injury Act („Vaccine Act“). This legislation establishes a National Vaccine Program „to achieve optimal prevention of human infectious diseases through immunization and to achieve optimal prevention against adverse reactions to vaccines.“ The Vaccine Act also institutes the National Vaccine Injury Compensation Program („NVICP“), a federal, no-fault compensation system which awards money to the victims of vaccine-related injuries and death. This paper describes the FDA’s role in ensuring the safety of vaccines, the civil litigation alternative to compensation, and the events leading up to the passage of the Vaccine Act. The paper, however, focuses on the NVICP, the actual operation of this compensation program, and the program’s effects on the compensation and prevention of adverse reactions to mandatory vaccinations. The paper also examines whether Congress’s goals in passing the Vaccine Act have been achieved and what reforms may be necessary in order to further these goals.

So why you need absolute immunity for liability if you have a amazing product that is 95% safe and effective?
1992
THE AIDS VACCINE: LEGISLATION TO LIMIT MANUFACTURER'S LIABILITY
https://digitalcommons.law.utulsa.edu/cgi/viewcontent.cgi?article=1949&context=tlr
1993
Returning to the timeline, in 1993, Bill Clinton was inaugurated as the 42nd President of the United States succeeding GHWB. The broader effort to remake.
Recommended Citation:
U.S. Congress, Office of “Technology Assessment, Proliferation of Weapons of Mass Destruction: Assessing the RiskOTA-ISC-559 (Washington, DC: U.S. Government Printing Office, August 1993).
NTIS order #PB94-107612 GPO stock #052-003-01335-5
1996
Defense Against Weapons of Mass Destruction
Act of 1996
1996 Congress and Special Weapons Nuclear, Chemical, Biological and Missile
1999
U.S. biosecurity began its first phases in 1999 during the Clinton presidency. That effort then transited to the 2000s and into the presidency of Bush 43 – George W. Bush.
First Annual Report
to
The President and The Congress of the
ADVISORY PANEL TO ASSESS DOMESTIC RESPONSE CAPABILITIES FOR TERRORISM INVOLVING WEAPONS OF MASS DESTRUCTIONI. ASSESSING THE THREAT
15 December 1999The following document is a reprint of the First Annual Report of the Advisory Panel to Assess Domestic Response Capabilities for Terrorism Involving Weapons of Mass Destruction, submitted to the President and the Congress on 15 December 1999. It is a report of the Advisory Panel, not a RAND publication. It was reviewed and edited by selected RAND professional staff, and was submitted for review and comment within the U.S. Government Interagency process.
Martin’s examination reveals that the first patent filings on coronavirus gene sequences with 99% identity to SARS-CoV-2 [the COVID-19 virus] and that were attributed to the claimed “novel” aspect of SARS-CoV-2, actually date back to 1999.
My Question is why does a gene sequences has anything to do with an Virus?
2000
28. Jan 2000 Timothy Miller, Sharon Klatfer, Albert Paul Reid and Elaine Jones filed what ultimately was issued as U.S. patent 6372224, which was the spike protein virus vaccine for the canine coronavirus, which is one of the multiple forms of coronavirus.
2001
Bioweapons research is banned
by an international treaty but nobody is checking for violations. This work includes bioengineering pathogens for medical research, techniques that also can be used to create deadly biological weapons. It’s an overlap that’s helped fuel speculation that the SARS-CoV-2 coronavirus was bioengineered at China’s Wuhan Institute of Virology and that it subsequently „escaped“ through a lab accident to produce the COVID-19 pandemic. Condemning the use of chemical and biological weapons as unacceptable under any context or circumstances, delegates urged all States to abide by critical existing international instruments for their regulation, as the First Committee (Disarmament and International Security) continued its general debate today.
MCAM was asked to monitor biological and chemical weapons treaty violations remembering the September 2001 anthrax events serving as part of an investigation that gave rise to the congressional inquiry into the anthrax origins and unusual activity around a potential anthrax drug treatment by Bayer; and throughout the fall of 2001, MCAM began to monitor an enormous number of bacterial and viral pathogens that were being patented through NIH, NIAID, U.S. AMRID [military] and a number of other collaborating international agencies.
March 2001 Tóth issued a 210-page „composite text“ designed to resolve some of the outstanding issues. Under the „composite text,“ several new aspects would be added to the biological weapons regime.
Liquid Computing
Imagine a computer, suspended in a flask of liquid, which assembles itself when the liquid is poured onto a desktop. Sound like science fiction? Hyman professor of chemistry Charles Lieber is making it happen in his laboratory, where researchers have already created tiny logic circuits and memory--the two main components of a computer--in just this manner. And these circuits are tiny, just a few atoms across.
https://www.harvardmagazine.com/2001/11/liquid-computing-html
Januar 2001 Smart Dust: Communicating with a Cubic-Millimeter Computer
The Smart Dust project is probing micro-fabrication technology’s limitations to determine whether an autonomous sensing, computing, and communication system can be packed into a cubic-millimeter mote to form the basis of integrated, massively distributed sensor networks. Brett Warneke, Matt Last, Brian Liebowitz, Kristofer S.J. Pister University of California, Berkeley.Funded by the Defense Advanced Research Projects Agency’s ( DARPA) Microsystems Technology Office, the Howard Hughes Doctoral Fellowship Program, and the Fannie and John Hertz Foundation
2001
THE ANTHRAX ATTACKS
Can you still remember on the anthrax attacks after the attacks on the
World Trade Center?
https://rumble.com/v2dqvkw-dr.-david-martin-anthrax-was-a-plan-to-pass-the-prep-act.html
Using anthrax as a weapon
Source: BBC News, October 17, 2001.
By Nick Caistor, BBC News Online
Military interest in the use of anthrax as a weapon began in the First World War. The Germans used it to contaminate animal feed and livestock but, unlike chemical gases, it was not employed directly against enemy troops.
The first mass use of anthrax spores as a weapon is said to have taken place during the Japanese occupation of China from 1932 to 1945.
The Japanese allegedly experimented with the use of anthrax and other biological weapons in Manchuria, and some 10,000 deliberately infected prisoners are thought to have died as a result.
Second World War
In the Second World War, the Germans did not launch the much-feared biological attack, although they and the Allied forces experimented with the possibilities of using anthrax or other agents.
The UK military tested spore delivery systems of anthrax on the tiny island of Gruinard off the Scottish coast.
These spores persisted and remained theoretically capable of infection for decades afterwards.
A massive decontamination effort, started in 1979 and completed in 1987, used 280 tonnes of formaldehyde and 2,000 tonnes of seawater to clean up the island.
Virulent strain
After the Second World War, the US continued its biological weapon research into the 1950s, when Iowa State University produced the virulent „Ames strain“ of anthrax which was later sold to many parts of the world.
In 1970, President Nixon ordered an end to the production of biological weapons in the United States, since when research there has been confined to developing means of defence against any biological attack.
In 1972, international concern led to a treaty banning the production and stockpiling of biological weapons. This was eventually signed by some 140 nations.
Although it was one of the treaty signatories, the Soviet Union continued researching and producing biological weapons – and in April 1979 an accidental release of anthrax spores from a military facility near Sverdlovsk caused 68 known deaths.
Gulf War
But the greatest fears that anthrax might be used as a weapon came during the 1991 Gulf War.
Iraq purchased anthrax spores from the United States in the 1980s, and was thought to be developing the capability to use them in warheads and in aerial attacks.
In the event, no biological weapons were used.
After the war, the UN Special Commission on Iraq (UNSCOM) destroyed the remaining production and stockpiling facilities for biological warfare in Iraq. „By 1998, we were able to establish that Iraq had no capability of producing biological weapons,“ a former UN inspector, Scott Ritter, told the BBC.
Anthrax powder
In the 1990s, the one publicised case of the use of anthrax for terrorist aims was by the Aum Shinrikyo group in Japan.
They are said to have tried unsuccessfully to release anthrax in Tokyo several times, leading them to change to sarin gas, with fatal results.
Producing large amounts of anthrax in powder form – necessary for its use as an effective large-scale weapon – is a complicated and expensive process.
It requires the use of large centrifuges for repeated washings, and then intensive drying to produce the concentrated or „military-grade“ powder.
The cost of this technology has led some experts in the United States to argue that the instigators of the present campaign must be a country, with previous experience, stocks and the necessary biotechnological expertise.
Scott Ritter also sees this as a real possibility.
„Terrorists do not have the facilities to weaponise anthrax, so if you have military grade anthrax being used, this would be the first solid evidence of state sponsorship,“ he said.
Source: https://web.archive.org/web/20210617030204/https://www.ph.ucla.edu/epi/bioter/anthraxasweapon.html
FBI Amerithrax conclusion
2007, after several years of scientific developments and advanced genetic testing coordinated by the FBI Laboratory, the Task Force determined that the spores in the letters were derived from a single spore-batch of Ames strain anthrax called “RMR-1029.” RMR-1029 had been created and maintained by Dr. Bruce E. Ivins at USAMRIID. This was a groundbreaking development in the investigation. It allowed the investigators to reduce drastically the number of possible suspects, because only a very limited number of individuals had ever had access to this specific spore preparation that was housed at USAMRIID. The Task Force then began applying traditional law enforcement techniques to a very limited universe.
https://vault.fbi.gov/Amerithrax/Amerithrax%20Part%2001%20of%2059/at_download/file
FDA – Bayer Corporation Letter for Approval for CIPRO
Cipro (Ciprofloxacin Hydrochloride) Tablets, IV Solution, IV in 5% Dextrose, IV in 0.9% Saline, and Oral Suspension
Company: Bayer Corporation
Application No.: 19-537/S38, 19-847/S24, 19-857/S27, 19-858/S21, & 20-780/S8
Approval Date: 8/30/2000https://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/19-537S038_Cipro.cfm
• Approval Letter(s) (PDF)
• Printed Labeling (PDF)
• Medical Review(s) (PDF)
• Pharmacology Review(s) (PDF)
• Statistical Review(s) (PDF)
• Microbiology Review(s) (PDF)
• Clinical Pharmacology Biopharmaceutics Review(s) (PDF)
• Administrative Document(s) (PDF)
• Correspondence (PDF)
All Roads Lead To Dark Winter
Anthrax War is a 2009 documentary filmabout the 2001 anthrax attacks and the rise of today’s biomilitary industrial complex that was co-produced by the Canadian Broadcasting Corporation and Arte-France. Broadcast internationally, it was nominated for the 2009 Prix Europa for Outstanding Current Affairs Broadcast. It also screened at the Frontline Club in London, the IDFA Festival in Amsterdam, the Tri-Continental Film Fest in Johannesburg, and the 9/11 Film Festival in Oakland, California, among other venues.
"The potential for creating and using biological weapons to wreak havoc is an urgent concern not just in America, but worldwide. In fact, many security experts believe that the next act of widespread terrorism will likely come from a weapon of biochemical means. In Anthrax: Bioterror as Fact and Fantasy, Philipp Sarasin explores the real threats of biological weapons--in contrast to the idea of biological substances as nebulous agents of terror--by analyzing the famous anthrax scares that occurred in the United States in 2001. Basing his analysis on government documents and media coverage between the events of September 11, 2001, and the beginning of the Iraq War in March 2003, he shows that the anthrax letters became the necessary fantasy-link between the 9/11 attacks and Saddam Hussein's "weapons of mass destruction." While many bioterrorism experts agree that it would be difficult to use anthrax effectively as a weapon in a large-scale attack, the anthrax scares that occurred in the wake of the September 11 terrorist attacks amplified the American public's fear and uncertainty about what might come next. In effect, these incidents infected the American psyche and created an increased sense of vulnerability that shaped the public's understanding of the War on Terror. Sarasin, in offering a European's view of the U.S. reaction to the anthrax scare, argues that while threats of bioterrorism are real, they are disproportionate to the fantasmal fears and illusions that now permeate American politics and culture. In short, fear of bioterror has contaminated modern American life."
https://archive.org/details/anthraxbioterror0000sara
Philipp Sarasin
https://cfront.org/udb/cproWpEQ2eBioterror.pdf
Analysis
https://www.angelfire.com/ny5/libertystrikesback/Rosenberg.html
Trump and the Israeli connection. (Johnny Gat Mirro9/11r)
https://archive.org/details/youtube-3yDtIeWOSN8
9 11, Israel, Trump Connection CASE CLOSED 2 (Johnny Gat Mirror)
https://archive.org/details/youtube-GnsZpOGXhog
9/11 119 Questions. Free Book. October 2019. | PDF | Aircraft Hijackings | Crime & Violence
2002
The CDC Patents the Coronavirus Transmissible to Humans
“The patent for that replication-defective coronavirus that attacks human lung cells was filed April 19, 2002 (Patent No. 7279327). “In other words, we made SARS,” Martin says. Or perhaps more accurately, Fauci and UNC did. Several months after that patent filing, the SARS outbreak in Asia occurred.”
https://www.m-cam.com/wp-content/uploads/2020/04/20200403_SARS_CoV_Patent_Corpus_Lit_Review.pdf
2003
„Efforts by the federal government to prepare for pandemic influenza at the national level include a $100 million DHHS initiative in 2003 to build U.S. vaccine production.
Several agencies within Department of Health and Human Services (DHHS) — including the Office of the Secretary,
the Food and Drug Administration (FDA), CDC, and the National Institute of Allergy and Infectious Diseases (NIAID) — are in the process of working with vaccine manufacturers to facilitate production of pilot vaccine lots for both H5N1 and H9N2 strains as well as contracting for the manufacturing of 2 million doses of an H5N1 vaccine.
2004
The enactment and early years of EUA
Ultimately, it was the War on Terror that would give rise to emergency use authorization. After the events of September 11, 2001 and subsequent anthrax mail attacks, Congress enacted the Project Bioshield Act of 2004. The act called for billions of dollars in appropriations for purchasing vaccines in preparation for a bioterror attack, and for stockpiling of emergency countermeasures. To be able to act rapidly in an emergency, Congress allowed FDA to authorize formally unapproved products for emergency use against a threat to public health and safety (subject to a declaration of emergency by HHS). The record indicates that Congress was focused on the threat of bioterror specifically, not on preparing for a naturally-occurring pandemic.
PUBLIC LAW 108–276—JULY 21, 2004 118 STAT. 835
https://www.congress.gov/108/plaws/publ276/PLAW-108publ276.pdf
2005
On October 27, 2005, the Department of Health and Human Services awarded a $62.5 million contract to Chiron Corporation to manufacture an avian influenza vaccine designed to protect against the H5N1 influenza virus strain. This followed a previous awarded $100 million contract to Sanofi-pasteur, the vaccines business of the Sanofi-aventis Group, for avian flu vaccine.
In October 2005, President Bush urged bird flu vaccine manufacturers to increase their production.[94]
On November 1, 2005 President Bush submitted a request to Congress for $7.1 billion to begin implementing the
National Strategy to Safeguard against the Danger of Pandemic Influenza. The request includes $251 million to detect and contain outbreaks before they spread around the world; $2.8 billion to accelerate development of cell-culture technology; $800 million for development of new
treatments and vaccines; $1.519 billion for the Departments of Health and Human Services (HHS) and Defense to purchase influenza vaccines; $1.029 billion to stockpile antiviral medications; and $644 million to ensure that all levels of government are prepared to respond to a pandemic outbreak.[96]
2006
According to The New York Times as of March 2006, „governments worldwide have spent billions planning for a potential influenza pandemic: buying medicines, running disaster drills, [and] developing strategies for tighter border controls“ due to the H5N1 threat.[83]
On 6 March 2006, Mike Leavitt, Secretary of Health and Human Services, said U.S. health agencies are continuing to develop vaccine alternatives that will protect against the evolving avian influenza virus.[97]
Synthetic Viral Genomics: Risks and Benefits for Science and Society
Ralph S. Baric – University of North Carolina at Chapel Hill
Cite as: Baric RS. 2006. Synthetic Viral Genomics.
In: Working Papers for Synthetic Genomics
Risks and Benefits for Science and Society, pp. 35-81. Garfinkel MS, Endy D, Epstein GL, Friedman RM, editors. 2007
https://dspace.mit.edu/bitstream/handle/1721.1/39652/Baric%20Synthetic%20Viral%20Genomics.pdf
2007
The National Security and Homeland Security Presidential Directive, signed on May 9, 2007 declares that in the event of a „catastrophic event“, George W. Bush can become what is best described as „a dictator“:
„The President shall lead the activities of the Federal Government for ensuring constitutional government.”
This directive gives the White House unprecedented dictatorial power over the government and the country, bypassing the US Congress and obliterating the separation of powers. The directive also placed the Secretary of Homeland Security in charge of domestic „security“ (terrorism).
(1) this directive establishes a comprehensive national policy on the continuity of Federal Government structures and operations and a single National Continuity Coordinator responsible for coordinating the development and implementation of Federal continuity policies.
This policy establishes „National Essential Functions,“ prescribes continuity requirements for all executive departments and agencies“, and provides guidance for State, local, territorial, and tribal governments, and private sector organizations in order to ensure a comprehensive and integrated national continuity program that will enhance the credibility of our national security posture and enable a more rapid and effective response to and recovery from a national emergency.
24.(b) „Catastrophic Emergency“ means any incident, regardless of location, that results in extraordinary levels of mass casualties, damage, or disruption severely affecting the U.S. population, infrastructure, environment, economy, or government functions.
B. World Health Organization (WHO) and U.N.
The World Health Organization (WHO) is a specialized agency of the United Nations (UN) that acts as a coordinating authority on international public health.
Established on 7 April 1948, and headquartered in Geneva, Switzerland, the agency inherited the mandate and resources of its predecessor, the Health Organization, which had been an agency of the League of Nations.The WHO’s constitution states that its objective „is the attainment by all peoples of the highest possible level of health.“
The WHO and UN will become the controlling agencies in the US in the event of a declared pandemic level 6.
The World Health Organization (WHO) has developed a global influenza preparedness plan, which defines the stages of a pandemic, outlines WHO’s role and makes
recommendations for national measures before and during a pandemic.
2009 Swine flu outbreak
In March and April 2009, an outbreak of a new strain of influenza commonly referred to as „swine flu“ infected many people in Mexico and other parts of the world.
The new strain was first diagnosed in two children by the CDC, first on April 14 in San Diego County, California and a few days later in nearby Imperial County, California.[78] Neither child had been in contact with pigs.
The outbreak was first detected in Mexico City, where surveillance began picking up a surge in cases of influenza-like illness (ILI) starting March 18.[80]
On April 18.[85] The Mexican cases were confirmed by the CDC and the World Health Organization to be a new strain ofH1N1.[80][86]
Cases were also reported in the states of San Luis Potosí, Hidalgo, Querétaro and Mexico State.[87] Mexican Health Minister José Ángel Córdova on April 24, said „We‘re dealing with a new flu virus that constitutes a respiratory epidemic that so far is controllable.“[87] Mexican news media speculate that the outbreak may have started in February near a Smithfield Foods pig plant amid complaints about its intensive farming practices,[88][89] although no pigs in Mexico have tested positive for the virus.[citation needed]
The first death from swine flu occurred on April 13, when a diabetic woman from Oaxaca died from respiratory complications.[91][92] The Mexican fatalities are alleged to be mainly young adults of 25 to 45.
Although by late April there had been reports of 152 „probable deaths“[94] in Mexico, the WHO had received reports of only 7 confirmed deaths as of April 29 and explicitly denied the larger figure.[95][96]
Mexico’s Health Secretary declared that around 100 early suspected deaths from swine flu could not be confirmed because samples were not taken.[5]
Cases were first discovered in the U.S. and officials soon suspected a link between those incidents and an earlier outbreak of late-season flu cases in Mexico. Withindays hundreds of suspected cases, some of them fatal, were discovered in Mexico, with yet more cases found in the U.S.and several other countries in the Northern Hemisphere.
Soon thereafter, the U.N.’s World Health Organization (WHO), along with the U.S. Centers for Disease Control and Prevention (CDC), expressed concern that the A(H1N1) could become a worldwide flu pandemic, and WHO then raised its pandemic disease alert level to „Phase 5“ out of the six maximum, as a „signal that a pandemic is at the imminent level“.
According to a Summary of latest H1N1 developments in the United States by Alexander S Jones May 19, 2009A) H1N1 may have killed an infant in New York who developed cyanosis with rapid progression to death. This is an ominous parallel to 1918. This suggests viral pneumonia, but we have no confirmation. Whether this is from the New York ‚consensus strain‘ or a new recombinant, mutant, or re-assortant is unknown at this time.
http://www.flutrackers.com/forum/showthread.php?t=105092
http://www.myfoxny.com/dpp/health/swine_flu/090519_second_possible_death_from_swine_flu_in_new_york_city
B) Dr. Niman has estimated there are currently 1 – 10 million infections in the United States. This matches my own assessment. With a case fatality rate of 0.1%, we can expect 1000 – 10000deaths — although it has become clear at this point the authorities are covering up the spread of the virus. With a case fatality rate of 0.4%, we can expect 4000 – 40000 deaths.
http://www.recombinomics.com/News/05180901/Swine_H1N1_Japan_6.html
C) H1N1 is rapidly spreading in schools. The articles I have pasted below are only the tip of the iceberg — this is across the country at this point.
Lowell had 123 students call in sick Monday and sent another 71 home with fevers and other flu-like symptoms the representative said
http://www.flutrackers.com/forum/showthread.php?t=105174
http://www.bizjournals.com/phoenix/stories/2009/05/18/daily24.html
The Dana Hall School in Wellesley has been shuttered for the next week after nearly 100 students and staff called in sick with fevers, sore throats, and other flu-like systems.
A spokeswoman for Dana Hall School in Wellesley said Tuesday there is no indication that swine flu is what prompted 90 students and eight faculty and staff members to call in sick on Monday, but the move was made after consulting with state and local public health officials.
A spokeswoman for the state Public Health Department says there are no confirmed swine flu cases at the school and no one associated with the school is being tested for the disease.
http://www.flutrackers.com/forum/showthread.php?p=235635#post235635
http://www.boston.com/yourtown/news/wellesley/2009/05/flu_closes_dana_hall_school_in.html
http://www.bostonherald.com/news/regional/view/2009_05_19_Wellesley_school_closes_after_rash_of_illnesses/srvc=home&position=recent
D) There has been a death from a possible lethal co-infection, a dangerous event suggesting worse is to come – see the case of the death from pneumonia of an oil platform worker who tested positive for multiple strains of the flu.
Possible Swine Flu Death in Little Rock
The death of a 28-year-old man in a Little Rock hospital over the weekend could be linked to the H1N1 virus better known as Swine Flu.
Reported by: KARK 4 News Monday, May 18, 2009
That’s according to Pulaski County Coroner Garland Camper, who tells KARK 4 that the man’s autopsy revealed he had suffered from more than one strain of flu. Camper calls that „somewhat unusual.“
Camper says the man was an offshore oil worker who had been in the hospital with flu-like symptoms, and had reportedly been ill for weeks.
http://arkansasmatters.com/content/fulltext/news/?cid=222431
E) Data has become available from case studies in California, from H1N1 hospitalizations.
15/25 patients have lung infiltrates, almost half have vomiting… this is somewhat disturbing.
The best predictive symptoms based on this data are:
Fever (97%)
Cough (77%)
Lung infiltrates (60%)
Vomiting (46%)
Shortness of breath (43%)
3 and #4 are unusual for influenza
http://www.flutrackers.com/forum/showthread.php?p=235601#post235601
F) An article in Science from last week estimated the H1N1 case fatality rate is 0.4% — four times higher than seasonal flu.
http://www.eurekalert.org/pub_releases/2009-05/icl-sfe051109.phpG) The ER in New York has become overwhelmed with patients — on Tuesday, seeing double the number of children who present with respiratory symptoms. Alan
D. Aviles, the president of the city‘s Health and Hospitals Corporation, said that emergency admissions were running about 50 percent higher than usual for adults and
―more than 100 percent above average‖ for children.
http://www.flutrackers.com/forum/showpost.php?p=235577&postcount=23
http://cityroom.blogs.nytimes.com/2009/05/19/toddlers-death-stokes-flu-concerns/?hp
„The first case was seen in Mexico on April 13. The outbreak coincided with the President Barack Obama‘s trip to Mexico City on April 16. Obama was received at Mexico‘s anthropology museum in Mexico City by Felipe Solis, a distinguished archeologist who died the following day from symptoms similar to flu, Reforma newspaper reported. The newspaper didn‘t confirm if Solis had swine flu or not. „
http://www.bloomberg.com/apps/news?pid=20601087&sid=aEsNownABJ6Q&refer=worldwideThe Paris-based World Organization for Animal Health (OIE) said April 27th that virus currently circulating in Mexico and the United States and which has killed at least 20 people had never been found before in any animal and was completely new.
„The virus has not been isolated in animals to date. Therefore, it is not justified to name this disease swine flu,“ the OIE said in a press statement.
The virus „includes in its characteristics swine, avian and human virus components,“ the OIE said, and urged that it be called „North American influenza,“ after its geographic origin.
The OIE said it was „urgent“ that is scientific research be carried out to determine the susceptibility of animals to what it said was a „new virus.“
The new strain is an apparent reassortment of four strains of influenza A virus subtype H1N1.[64] Analysis by the CDC identified the four component strains as one endemic in humans, one endemic in birds, and two endemic in pigs (swine).
Alexander S Jones, former employee the NIH, has analyzed the genome sequence of the virus and concluded „we must seriously consider a laboratory origin for this virus“.
BLAST sequence homology of ’swine flu‘ indicates both the Hemagglutinin (HA) surface protein as well as the Non-structural (NS1) interferon Inhibition proteins are novel recombinants previously unidentified in nature.
Both these influenza proteins, based on the genetic sequences released Friday May 1st by the U.S. Centers of Disease Control (CDC), share their closest genetic identity with turkey (avian) and pig (swine) strains from multiple continents including North America as well as Asia. Even the closest matches indicate 5% previously unidentified genetic material.
I submit this evidence, coupled with the lack of the presence of this virus at the pig farm near the proposed CDC’s „patient zero“ (a 5 year old from La Gloria, 80km away from the pig farm in Perote, Mexico), shows that the origin of the flu outbreak remains unidentified at this time, and cannot be ascribed to Mexican or North American swine.
Furthermore, I submit that since 5% of both these influenza A RNA sequences share no known homology in any public
databases (in addition to the avian/swine hybrid nature of both these critical genes), that we must seriously consider a laboratory origin for this virus.
Future research that may be promising includes identifying critical SNPs, especially in the PB2 and the NS1 coding regions which may be markers for evolution of pathogen virulence, and should be closely monitored. The hem-agglutinin protein should also be monitored for acquisition of a poly-basic amino acid site which would give the viruspantrophic properties as in the 1918 pandemic. ―(Alexander S Jones)
The World Health Organization on May 11 2009 said leading vaccine producers including Baxter, Novartis, GlaxoSmithKline and Sanofi-Aventis had requested „wild type virus“ samples of the A (H1N1) or swine flu virus. MedImmune, which is now part of AstraZeneca, Baxter, CSL and Solvay are also being sent samples, as are smaller
developers Microgen, Nobilon International, Omnivest Vaccines and Vivaldi. The WHO is co-coordinating scientific discussions over the virus, and has said that, within the next few weeks, it is likely to make a recommendation on
whether and how to produce a pandemic vaccine.Latest Pandemic Time Estimates, based on Los Alamos Flu Simulation
http://www.lanl.gov/news/images/bird4x3red.mov
Thursday July 16
(by Day 87, aka by Thursday July 16th) Day 87 <– Wave 1 Outbreak Peaks Wave 2 Outbreak Peaks at +90 days, so approx mid October
Monday June 8
(by Day 53, aka by Friday June 12th)
Day 49 <– WHO Declares Phase 6Monday June 22 Day 63 <– Cases Go Exponential
(by Day 60, aka by Friday June 19th)
III. Evidence the swine flu vaccines are bioweapons
The „bird flu“ has been classified by the United States government in its own export regulations as a biological weapon, and there are grounds for believing the ―swine flu‖, likewise, is a bio-engineered virus and a component of a biological weapons system as defined by Section 175 (a) of BWATA designed, like the ―bird flu‖, to deliver toxins and microorganisms so as to deliberately inflict disease on death on people while being disguised as injections for prophylactic, protective, or other peaceful purposes.
Commerce Department regulations supplement listing pathogens whose vaccines are subject to export restrictions for countries classified as sponsors of terrorism (see pages 57-60, 70)
http://www.access.gpo.gov/bis/ear/pdf/ccl1.pdfThe United States bars the export of vaccines for the bird flu, smallpox, yellow fever, and many other pathogens to five countries classified as sponsors of terrorism.
Under Department of Commerce rules, a long list of vaccines for viruses, bacteria, and biological toxins cannot be exported to Cuba, Iran, North Korea, Sudan, and Syria unless they obtain a special export license, which can take weeks.
The list of pathogens subject to the rules includes viruses that cause dengue fever, Ebola fever, Marburg fever, Rift Valley fever, and monkeypox. A list of animal pathogens covered by the restrictions includes highly pathogenic bird
flu viruses. Bacterial pathogens on the restricted list include anthrax and the microbes that cause tularemia and plague. Not on the list are the causes of common vaccine-preventable diseases, such as measles, mumps, rubella, chickenpox, and seasonal influenza.
The Associated Press reported: ―Deep inside the United States export regulations is a single sentence that bars U.S. exports of vaccines for avian bird flu and dozens of other viruses to five countries designated „state sponsors of terrorism.“
http://news.yahoo.com/s/ap/20081011/ap_on_re_as/as_bird_flu_biological_warfare;_ylt=An9WoLAijbbjeNwhYV6N98Ws0NUEUS controls bird flu vaccines over bioweapon fears
By ROBIN McDOWELL, Associated Press Writer Sat Oct 11, 7:14 AM ET
When Indonesia’s health minister stopped sending bird flu viruses to a research laboratory in the U.S. for fear Washington could use them to make biological weapons, Defense Secretary Robert Gates laughed and called it „the nuttiest thing“ he’d ever heard.Yet deep inside an 86-page supplement to United States export regulations is a single sentence that bars U.S. exports of vaccines for avian bird flu and dozens of other viruses to five countries designated „state sponsors of terrorism.“
The reason: Fear that they will be used for biological warfare.
So, the United States government views vaccines as tools of biological warfare, giving indirect confirmation to the fears of the Indonesian Health Minister.
Furthermore, Ex-HHS Secretary Mike O. Leavitt refused to provide BIRD FLU VACCINES created by contract with Sanofi-Pasteur to rogue „terrorist“ nations like Iran, North Korea, and Syria solely because the VACCINE could be used
as a „BIOLOGICAL WEAPON“ by „terrorist nations“. (See http://crooksandliars.com/node/23360/print)
Leavitt recently declared that a pandemic is „nature’s terrorist“.(See http://news.yahoo.com/s/ap/20090509/ap_on_he_me/med_swine_flu_pivotal_moments) and
http://www.federalnewsradio.com/nid=35&sid=1670164.Here we have ex-HHS secretary Leavitt, declaring that a pandemic is a useful form of „terrorism“.
Since untested, untried, and potentially lethal
„experimental vaccines“ are restricted as „biological weapons“ from distribution to „rogue nations“, why even contemplate forcing the same „vaccine“ onto American citizens?
The only purpose for forcing American citizens to take these vaccines can be to cause death and injury under the guise of employing them for peaceful purposes because these vaccines are according to the United States government‘s
own regulations so dangerous they have to be kept out of the hands of ―terrorist nations‖ for fear they might use them in a terrorist attack.
Any group of American, dual- American citizens or citizens of other countries who knowingly develops, produces, stockpiles, transfers, acquires, retains, or possesses any biological agent, toxin, or delivery system for use as a
weapon against the people of America, or knowingly assists a foreign state or any organization to do so, also employing deceit and fraudulent misrepresentation violates BWATA (see Attachment 1).Section 175: Prohibitions with respect to biological weapons
(a) IN GENERAL- Whoever knowingly develops, produces, stockpiles, transfers, acquires, retains, or possesses any biological agent, toxin, or delivery system for use as a weapon, or knowingly assists a foreign state or any
organization to do so, shall be fined under this title or imprisoned for life or any term of years, or both. There is extraterritorial Federal jurisdiction over an offense under this section committed by or against a national of the United States.‖
The Act broadly defines several terms related to biological warfare of vector, toxin, biological agent and delivery system.
The ―swine flu‖ virus fits the BWATA definition of a biological agent to be classified as a bioweapon as:any micro-organism, virus, infectious substance, or biological product that may be engineered as a result of biotechnology, or any naturally occurring or bioengineered component of any such microorganism, virus, infectious substance, or biological product, capable of causing death,
disease, or other biological malfunction in a human, an animal, a plant, or another living organism; deterioration of food, water, equipment, supplies, or material of any kind or deleterious alteration of the environment
The ―swine flu‖ has killed and injured people in the United States alone and so meets the BWATA of a toxin:
―Toxin: „whatever its origin or method of production – – any poisonous substance produced by a living organism; or any poisonous isomer, homolog, or derivative of such a substance“.
The forced injections of the population of toxins under guise of offering prophylactic treatment are the delivery system as defined by BWATA and the vaccination process itself will release a fully weaponized virus:
―Delivery system: „any apparatus, equipment, device, or means of delivery specifically designed to deliver or disseminate a biological agent, toxin, or vector“.Constituting the vector as defined by BWATA are the people of the United States who will be injected by force en masse with disease producing microorganisms, and so allow the
virus to mutate and develop into more lethal strains.
―Vector: „a living organism capable of carrying a biological agent or toxin to a host“.‖IV. Scientific evidence the ―swine flu‖ virus is an
artificial (genetic) virus.Evidence comes from the Paris-based World Organization for Animal Health (OIE), which said on April 27th the virus currently circulating in Mexico and the United States and which has killed at least 20 people has never been found in
any animal.
„The virus has not been isolated in animals to date.
Therefore, it is not justified to name this disease swine flu,“ the OIE said in a press statement.
The virus „includes in its characteristics swine, avian and human virus components,“ the OIE said, and urged that it be called „North American influenza,“ after its geographic origin.
The OIE said it was „urgent“ that scientific research be carried out to determine the susceptibility of animals to what it said was a „new virus.“
Also, Adrian Gibbs, the Australian virologist, who was one of the first to analyze the genetic construction of the swine flu virus, and who was part of the team which developed anti-flu vaccines Tamiflu and Relenza, believes the disease – which has spread across the world in recent weeks – was made in laboratories.
Gibbs and two colleagues analyzed the publicly available sequences of hundreds of amino acids coded by each of the flu virus‘s eight genes. He said he aims to submit his three-page paper today for publication in a medical journal.The World Health Organization is investigating a claim by an Australian researcher that the swine flu virus circling the globe may have been created as a result of human error, according to a report on May 13 (Bloomberg) —
http://www.bloomberg.com/apps/news?pid=20601124&sid=aShZig0Cig4g.Andrew Rambaut, a viral geneticist at the University of Edinburgh, has said: ―The new neuraminidase gene that came in from Eurasian swine is one we‘ve never before seen circulating in humans.
―This is what we call a reassortment between two currently circulating pig flu viruses,‖ he said. ―Why it‘s emerged in humans is anyone‘s guess. It hasn‘t been seen before in pigs as far as I know.
http://www.wired.co.uk/news/archive/2009-04/29/swine-flu-genes-from-pigs-alone.aspx
Dr True Ott has reported that the published definition of the swine flu by the NCSL is identical to Jeffrey Taubenbergers 1997 initial findings concerning the 1918 killer virus which he successfully resurrected 6 years later.
It easiest to explain this highly improbable match between the two viruses by assuming the „swine flu― virus was deliberately, and systematically engineered to resemble the 1918 Spanish killer flu virus.
Dr Ott explains that Taubenberger‘s initial 1997 report identified the 1918 killer virus as a „novel“ (new) swine flu that „recombined“ avian (H5N1) as well as human (H3N2) virus fragments in its RNA structure.
Taubenberger, so Dr Ott argues, then used a complex computer program to perfectly match the RNA and DNA structures, in order to replicate and „resurrect“ the 1918 killer Spanish flu virus as a powerful biological weapon.By A. True Ott, PhD, ND
The „Spanish“ influenza pandemic killed at least 20 million people in 1918-1919, making it the worst infectious pandemic in history.
Understanding the origins of the 1918 virus and the basis for its exceptional virulence may aid in the prediction of future influenza pandemics. RNA from a victim of the 1918 pandemic was isolated from a formalin-fixed, paraffin-embedded, lung tissue sample. Nine fragments of viral RNA
were sequenced from the coding regions of hem-agglutinin, neuraminidase, nucleoprotein, matrix protein 1, and matrix protein/2. The sequences are consistent with a novel H1N1 influenza a virus that belongs to the subgroup of strains that infect humans and swine, not the avian subgroup.
SOURCE: Science Magazine Report, 21 March 1997, Dr. Jeffrey Taubenberger et. al. See
http://www.sciencemag.org/cgi/content/abstract/275/5307/179https://www.nytimes.com/1997/03/21/us/genetic-material-of-virus-from-1918-flu-is-found.html
Taubenberger‘s initial report identified the 1918 killer virus as a „novel“ (new) swine flu that „recombined“ avian (H5N1) as well as human (H3N2) virus fragments in its RNA structure. Taubenberger used a complex computer program to perfectly match the RNA and DNA structures, and then successfully replicated and „resurrected“ the 1918 killer flu as a powerful biological weapon in 2003, 6 years later.Now, indeed as Taubenberger foresaw in 1997, evil and conspiring men in positions of high power can not only PREDICT FUTURE INFLUENZA PANDEMICS, but they can also UNLEASH THEM AT WILL from laboratory test tubes in order to achieve socio-economic agendas.
It should concern EVERY MAN, WOMAN, AND CHILD in America (as well as the entire world) that according to the World Health Organization
(WHO) and the Centers for Disease Control (CDC) in Atlanta, Georgia, the so-called ―Swine Flu infecting and killing human beings in Mexico and North America this spring and summer, is ―a new subtype of the A/H1N1 not previously detected in swine or humans. This novel H1N1 influenza (swine flu) virus is a triple recombinant including gene segments of human, swine, and avian origin. Source:http://www.ncsl.org/?tabid=17089
(Interestingly, the National Council of State Legislatures (NCSL) is an unelected bureaucracy of policy-makers instigated and promulgated
by Utah‘s Dixie Leavitt, the father of Mike O. Leavitt the PANDEMIC FLU GURU of the Bush administration.)
This published definition by the NCSL is IDENTICAL to Taubenbergers 1997 initial findings concerning the 1918 killer virus which he successfully resurrected 6 years later.Is this just a bizarre, meaningless coincidence? You decide.
http://www.unfictional.com:80/pdf/baxter-genocide-bioweapons.pdfJohn D. Rockefeller labs and factories in China
produced these Typhus vaccines in 1916 by harvesting pus from infected humans, injecting the infectious matter into pig hosts, then mixing the harvested contaminants into chicken egg albumin to be injected into human hosts as a
„vaccine“.
Rockefeller, always a shrewd businessman, supplied both sides, (German as well as Allied armies) with his toxic and lethal vaccine brew. Immediately after vaccination, many soldiers fell ill with what was called at the time „Para-Typhoid“infection — i.e. nausea, vomiting, diarrhea, and killing pneumonia. Subsequent waves spread across the globe, killing as many as 50 million innocent souls worldwide.
(Source: The Horrors of Vaccination – Higgins, 1921)
Only much later did the world‘s medical establishment wrongfully label and name the deadly recombinant virus accidentally spawned by Rockefeller‘s vaccine the „1918 Spanish Flu“. Of course, Rockefeller‘s multi-billion dollar
pharmaceutical empire could not afford to label it what it really was: „Vaccine-Induced Disease of 1918“.

day, the stage is set for eugenics and genocide on a truly massive scale. The Taubenberger Frankenstein monster has been released and hundreds of millions of 1918 influenza vaccine serums have been produced.
It was an accident in 1918, however the subsequent cover-up is/was unconscionable. What is occurring now is inexcusable and criminal in the extreme.Mother Nature does not ―naturally‖ recombine bird, swine and three human influenza viruses. (Birds do not exchange bodily fluids with pigs and humans in un-natural sexual liaisons — only sick, warped scientists can create such a monstrosity.)
Mexico’s top government epidemiologist said Wednesday that it is „highly improbable“ that a farm in the Mexican state of Veracruz operated by Smithfield Foods Inc. is responsible for the nation’s swine-flu outbreak.Miguel Ángel Lezana, the government’s chief epidemiologist, said in an interview that pigs at the farm are from North America, while the genetic material in the virus is from Europe and Asia.
http://online.wsj.com/article/SB124105320874371313.html
Dr Leonard Horowitz states in a 10.41 minute YouTube clip that the swine-bird-human flu strain in Mexico could have only come from Dr James S Robertson and colleagues because:
„nobody else takes H5N1 Asian-flu infected chickens, brings them to Europe, extracts their DNA, combines their proteins with H1N1 viruses from the 1918 Spanish flu isolate, additionally mixes in some swine flu genes from pigs, then
reverse engineers them to infect humans.“
In addition, Dr Horowitz indicates that there is hard evidence to show that Dr James Robertson believes it is OK to prime populations worldwide by releasing viruses he and his colleagues are creating in advance of a pandemic.
Dr Horowitz mentions the involvement of Dr Rick Bright who has ties to the WHO, the CDC and Novovax Inc, and is involved in PATH – Influenza Vaccine Project in the Vaccine Development Global Program.An analysis of the ―swine flu genome sequence by Alexander S Jones indicates that 5% of both these influenza A RNA sequences share no known homology in any public databases (in addition to the avian/swine hybrid nature of both these
critical genes), and so a laboratory origin for this virus must be seriously considered.
„Influenza A virus“ (A/Texas/04/2009(H1N1)) segment 8 nuclear export protein (NEP) and nonstructural protein 1 (NS1) genes, complete cds
http://www.ncbi.nlm.nih.gov/nuccore/FJ981620
HA („hemagglutinin“) protein BLAST sequence homology
(Page 32 – 45) http://www.unfictional.com:80/pdf/baxter-genocide-bioweapons.pdf
Influenza A virus (A/Swine/Minnesota/5)
Alexander S Jones concluded „we must seriously consider a laboratory origin for this virus“ because 5% of both these influenza A RNA sequences share no known homology in any public databases.
„BLAST sequence homology of ’swine flu’“ indicates both the Hemagglutinin (HA) surface protein as well as the Non-structural (NS1)
interferon Inhibition proteins are novel recombinants previously unidentified in nature.
Both these influenza proteins, based on the genetic sequences released Friday May 1st by the U.S. Centers of Disease Control (CDC), share their closest genetic identity with turkey (avian) and pig (swine) strains from multiple continents including North America as well as Asia. Even the closest matches indicate 5% previously unidentified genetic material.
I submit this evidence, coupled with the lack of the presence of this virus at the pig farm near the proposed CDC’s „patient zero“ (a 5 year old from La Gloria, 80km away from the pig farm in Perote, Mexico), shows that the origin of the flu outbreak remains unidentified at this time, and cannot be ascribed to Mexican or North American swine.
Furthermore, I submit that since 5% of both these influenza A RNA sequences share no known homology in any public databases (in addition to the avian/swine hybrid nature of both these critical genes), that we must seriously consider
a laboratory origin for this virus.
Future research that may be promising includes identifying critical SNPs, especially in the PB2 and the NS1 coding regions which may be markers for evolution of pathogen virulence, and should be closely monitored. The hemagglutinin protein should also be monitored foacquisition of a poly-basic amino acid site which would give the virus pantrophic properties as in the 1918
pandemic.Virologist Adrian Gibbs said that the „swine flu“ was leaked from a lab and, interestingly, Baxter has large-scale production and research facilities close to Mexico City, where the outbreak of the „swine flu“ occurred.
The „mysterious origin“ of the swine flu was underlined by the Mexico‘s Chief Epidemiologist M.A. Lezana, who said that among the first mortalities was a Bangladeshi born street vendor in Mexico City who fell ill in early April.
The man is said to have met his brother in Merida, Yucatan in early April and returned to Mexico City before he died.
The assertion is that the brother, a Bangladeshi or a Pakistani, was also ill.
(http://ahrcanum.wordpress.com/2009/05/05/baxter-pharmaceutical-plant-in-mexico-ground-zero-for-flu-outbreak/)
Edgar Hernandez of La Gloria fell ill with a fever and headache in early April according to his mother Maria del Carmen Hernandez. His mom took him for healthcare, and he recovered swiftly. The Financial Times timeline says it was April 2.Mexican officials confirm that Edgar Hernandez did carry the A/H1N1 virus, but they have not confirmed any other resident did or does. No one else in Edgar‘s family got sick at all. A state public health doctor says, “We just don’t know how he (Edgar) got sick. Maybe it was a genetic accident of some kind.”
Also, the Financial Times timeline points to a La Gloria health official requesting assistance in February for an outbreak of an acute respiratory disease; and on April 6 there was a health alert in La Gloria with 400 seeking medical treatment.
How did Edgar Hernandez become positive if not for the pigs of La Gloria?
And why cannot Smithfield find the A/H1N1 in
one million pigs — all of whom will be slaughtered soon enough unless that Bangladeshi subplot fleshes out. More soon.
One thought from
http://www.naturalnews.com/026141.htmlnotes, it is astonishing to realize, because for this to have been a natural combination of viral fragments, it means an infected bird from North America would have had to infect pigs in Europe, then be re-infected by those same pigs with an unlikely cross-species mutation that allowed the bird to carry it again, then that bird would have had to fly to Asia and infect pigs there, and those Asian pigs then mutated the virus once again (while preserving the European swine and bird elements) to become human transmittable, and then a human would have had to catch that virus from the Asian pigs — in Mexico! — And spread it to others in order to assist the World Health Organization in developing a new vaccine, reaping billions in the process. ‖
Just 50 miles from the H1N1 ground zero outbreak in Mexico City, lies Baxter‘s manufacturing plant in Cuernavaca, Mexico. It was named one of the 10 Best Plants in North America for 2008 by Industry Week magazine. http://www.baxter.com/about_baxter/news_room/news_releases/2008/12_19_08_industryweek.html
The plant manufactures, ―Water for Injection, Devices Medical, Premixes Formulations,‖ according to http://www.alibaba.com/member/juanbaxter/aboutus.html.
What else do they manufacture there?What kind of water gets injected?
Germ Warfare?
Cures or Causes?
Baxter was also responsible for the mislabeled, recalled doses of Heparin. Baxter recalled one lot of a product that hospitals use to treat burn victims and patients in shock after a test found a rare form of HIV in the plasma used to make the product. HIV-2 in plasma!
http://www.aegis.org/news/ct/2001/CT010716.html. Baxter also manufactures a vaccine against tick-borne encephalitis (TBE) and a vaccine against group C meningococcal meningitis.http://www.baxtervaccines.com/?node_id=312
,
in addition to other pharmaceutical products, anesthetic‘s, pumps, etc.Bio Hazards? Virus Mutations?
Vaccines? Cures or Causes?
Baxter was also responsible for the mislabeled, recalled doses of Heparin. Baxter recalled one lot of a product that hospitals use to treat burn victims and patients in shock after a test found a rare form of HIV in the plasma used to make the product. HIV-2 in plasma!
http://www.aegis.org/news/ct/2001/CT010716.html. Baxter also manufactures a vaccine against tick-borne encephalitis (TBE) and a vaccine against group C meningococcal meningitis.http://www.baxtervaccines.com/?node_id=312
in addition to other pharmaceutical products, anesthetic‘s, pumps, etc.
http://www.ecomm.baxter.com/ecatalog/browseCatalog.do?lid=1
0001&cid=10016
The National Autonomous University of Mexico (UNAM) has a satellite campus located in Cuernavaca, which is aimed at research and graduate studies. It also has an undergraduate program in genomics.
Cuernavaca is the home of the following research centers: Center for Genomic Sciences (UNAM),[3] the Institute of Biotechnology (UNAM),[4] the Institute of Physical Sciences (UNAM),[5] the Center for research in Energy (UNAM), the Institute of Mathematics (UNAM), the Center for Research in Engineering and Applied Sciences (UAEM),[6] and the National Institute of Public Health. Cuernavaca has the highest concentration of scientists and researchers in Latin America.-WIKI http://en.wikipedia.org/wiki/Cuernavaca Cuernavaca is certainly a who‘s who in genetics and research.
Since President Obama visited Mexico on April 16, the virulent flu has stricken more than 1,000 people, killing nearly 70 of them, including one person the President met at a museum.
Obama was received at Mexico‘s anthropology museum in Mexico City by Felipe Solis, a distinguished archeologist who died the following day from symptoms similar to flu, Reforma newspaper reported.
http://www.bloomberg.com/apps/news?pid=20601087&sid=aEsNown
ABJ6Q&refer=home
A federal agent who traveled to Mexico with President Obama this month probably contracted swine flu and infected several members of his family in Anne Arundel County, prompting assurances yesterday from the White House that the president was safe.
FDA’s newfound EUA authority would be used relatively sparingly for the first 16 years following its enactment. During that time, its most extensive use was in combating the H1N1 swine flu pandemic of 2009 by authorizing medical equipment and existing influenza drugs. Health policy experts would look back on the use of EUA against H1N1 as an overall success. It would also be used (pursuant to an amendment allowing for preemptive EUAs) to authorize occasional countermeasures in anticipation of MERS, Ebola, Zika, and other epidemics, none of which ultimately materialized in the United States.
In 2009, a group of Epstein-Ghislaine aligned billionaires discussed overpopulation & how to curb population growth.
2010
The Rockefeller Plan (The Fourth Industrial Revolution Plan, The Great Reset Plan) was created calling for “A world of tighter top-down government control and more authoritarian leadership (including: Quarantines, curfews, lockdowns and forced vaccination) – See Page 16
https://timetofreeamerica.com/wp-content/uploads/Rockefeller-Foundation-1.pd
2011
The now convicted Harvard professor Charles Lieber (Who Was Paid $50,000 Per Month Illegally by the Chinese Communist Party) created nano-technology that allows human cells to send and receive signals.
• https://www.harvardmagazine.com/2011/01/virus-sized-transistors
• https://stateofthenation.co/?p=60567
2012
In 2012, Epstein held a “doomsday” conference consisting of many of the or aligned scientists.
https://www.pr.com/press-release/403599 Think about that… the bulletin of scientists doomsday conference in 2012 organized by Jeffrey Epstein in the Virgin Islands.
https://www.nytimes.com/2019/07/21/business/media/jeffrey-epstein-media.html
Billionaire financier weighs in on the future of Bitcoin https://thenextweb.com/news/jeffrey-epstein-bitcoin
And in a speech at the UN council, GhiselineMaxwell Ocean leaders and the TerraMar Project founder tell the world why an ocean-specific sustainable development goal at the United Nations is essential to healthy oceans for generations to come.“TerraMar”
t
https://www.pr.com/press-release/403599
Bill Gates had Dr Melanie Walker (who was previously Epstein’s scientist) planted at World Bank
She reported to President Jim Kim
Jim Kim was previously at WHO, before Obama appointed him to World Bank President
While at WHO, Jim Kim signed a partnership between WHO & Clinton Foundation, with Ira Magaziner as the signee
The CEO of Clinton Health Access Initiative, Ira Magaziner, was flying on Epstein’s plane when making some of these arrangements
https://www.clintonfoundationtimeline.com/main-timeline/
Melanie is married to former Microsoft exec, Steve Sinofsky. Microsoft was also infiltrated by the Maxwell sisters via CommTouch
WEF tech pioneer, Isabel Maxwell, cofounded Isreal Venture Network with Clinton’s PITAC Benhamou
Melanie Walker went from Epstein, to
WHO, to Gates, to World Bank
During Melanie’s time at the Gates Foundation, BMGF had partnered with
on Belt & Road initiative Healthcare, and BGI on **health** & **Agriculture**. WHO’s Ren Minghui
was involved with Uighurs, belt & road *One Health*, and “family planning”
Chelsea Clinton sits on the board of “Partners in Health”. Jim Kim cofounded Partners in Health
Ira Magaziner also flew with Epstein/Clinton in 2003, to Beijing. Shortly after, the Clinton Foundation partnered with Biomerieux
The stepson of Robert Maxwell’s attorney, Antony Blinken, appointed Epstein-flying CIA Gayle Smith, to be the global vaccine coordinator for Biden. Gayle also served as USAID administrator (would have seemingly had a role in money going to EcoHealth, WIV, Metabiota). Upon being appointed in the Biden admin, she provided billions through COVAX, to GAVI
Metabiota Nathan Wolfe was tied to Ghislaine&epstein…and ecohealth-OneHealth-PlanetaryHealth
GAVI was formed between Gates Foundation & WEF… in 1999
Chelsea Clinton wrote a paper on the Global Fund, which highlights some of these ties
Epstein scientist, George Church, had been working with
BGI, as well as Personal Genome Project, while at Harvard. Epstein founded/funded Harvard’s Program for Evolutionary Dynamics
WHO’s Tedros, from Ethiopia, appears to have been recruited by Gayle Smith. He cofounded IHME, ran at Melanie Walker’s University of Washington
IHME provided covid forecasting for much of the world…including imperial college of London. Prince Andrew met with Xi, Epstein (with Melanie Walker), Peter nygard, and Imperial College worked with
Biomerieux groomed Moderna CEO, who got a DARPA contract upon going to Moderna. Biomerieux worked with Ren Minghua to plan uighurs “healthcare”. Biomerieux played a role in covid testing, with BioFire
Larry Summers wrote a chapter on global disease planning while at world bank. He advocated for
into World Trade Organization. After serving in Clinton Admin, he gave Epstein access to top scientists at Harvard, as Harvard President. Larry is on the board of “The One Campaign” with Gayle Smith
World Bank has promoted changing global finance to coincide with Lynn Rothschild’s Inclusive Capitalism… NACs, SASB, ESG, DEI, etc all have
Gates advantages, and stem from World Bank…JingDong Hua worked on these at World Bank with Melanie. Lynn was highly involved with Epstein & Ghislaine. She was on the board of First Mark with Microsoft’s Nathan Myhrvold & Kissinger
Epstein flying Eric Nonacs, specializing in NGOs & Pandemics, helped Epstein cofound Clinton Foundation
WEF was founded on Club of Rome Malthusian ideology. WEF board of Trustees Gore was named on Epstein’s island. Gore, Gates, and many of these people are/were club of Rome
AGENDA21 was founded on Rio Earth Charter, coauthored by
Maurice Strong & Gorbachev. Gorbachev & Robert Maxwell tried to cofound a joint science foundation. Robert Maxwell monopolized scientific journals. Gorbachev wanted to use “climate” for global governance
Bill Barr’s dad, Don, from OSS, recruited Epstein to teach at Dalton. Antony Blinken came from Dalton
SoS Clinton had Isabel Maxwell’s nephew in state Dep
Epstein/Maxwell operations = global eco & health tyranny
https://x.com/JesseMatchey/status/1472957015584436225
Who was meeting with Prince Andrew (think the Royals pushing Terra Carta)?
Underpinning the rewriting of global finance
No one is interested?
A planetary emergency
Co-conspirator of Jeffrey Epstein; Daughter of disgraced British newspaper owner Robert Maxwell
Source documents
Show 102550100 entriesSearch:
DocumentUpdated
2024-07-17 Prosecution rests in Ghislaine Maxwell trial
2024-01-18 Shutterstock / Nicky Haslam and Ghislaine Maxwell
2024-01-07. Ghislaine Maxwell Breaks Silence on Impending Release of Epstein Names
2024-01-03 “CLINTON AND GHISLAINE BECAME SUPER CLOSE”: AS THE EPSTEIN SCANDAL SPIRALS, A NEW FOCUS ON OLD NAMES
2023-11-29 Photo of Neil deGrasse Tyson at a party a Maxwell’s house
2023-11-16 Elon Musk photo with Ghislaine Maxwell
2023-10-28. Ex-Reddit CEO tweeted she ‘knew about’ Ghislaine Maxwell allegations in 2011
2023-10-28 Ghislaine Maxwell’s Own Lawyers Are Now Suing Her
Ghislaine Maxwell’s mysterious hubby Scott Borgerson, once seduced by her power, now MIA2021-12-12
Ghislaine Maxwell, Epstein’s Companion, Goes on Trial 2021-11-16
Ghislaine Maxwell’s brother reveals key detail about notorious Prince Andrew photo2021-03-12wikipedia2021-01-04mintpressnews.com – Former Israeli Intel Official Claims Jeffrey Epstein, Ghislaine Maxwell Worked for Israel2020-12-06Ghislaine Maxwell’s legal team includes ‚El Chapo‘ prosecutor2020-07-29
Is Dr. Fauci Married to Ghislaine Maxwell’s Sister? 2020-07-09
United States v. Maxwell (1:20-cr-00330) District Court, S.D. New York 2020-07-09
dockets.justia.com – Farmer v. Indyke et al 2020-02-16
2020-01-13 nymag.com – The Fantasist
Jeffrey Epstein’s alleged madam Ghislaine Maxwell was a guest at a Jeff Bezos-hosted retreat in 20182019-11-02
The one weird court case linking Trump, Clinton, and a billionaire pedophile2019-07-08
FEC Filing 249815456652019-07-08
FEC Filing 249815456662019-07-08
FEC Filing 279315533472019-07-08
The Independent – It’s the company you keep… the Duke’s dangerous liaisons2019-01-25
2002 NYS Board of Elections Financial Disclosure Report: Periodic July2017-10-23
FEC contribution search 2016-07-06
Daily Mail – Unsavoury association: How Robert Maxwell’s daughter ‚procured young girls‘2016-05-29
New York Magazine – Jeffrey Epstein: International Moneyman of Mystery2016-02-27
I Tried to Warn You About Sleazy Billionaire Jeffrey Epstein in 2003 2015-01-07
FEC Filing 24962531143 2015-01-07
FEC Filing 24962531144 2015-01-07
Epstein documents allege sex tapes of Clinton, Prince Andrew and Richard Branson
Thread by @AliAdair22 on Thread Reader App
Nine People Who Could Be Running Scared After Jeffrey Epstein's Arrest
SHOCKING new Epstein papers reveal CREEPY targeting of children for medical experiments | Redacted
Lolita-Express: Rockefellers Deep Entanglement mit Jeffrey Epstein
The Ghislaine Maxwell Trial-Closing Arguments, Leah Saffian of the Infamous Burger Leak Present?
Gunman Attacks House of Judge Assigned to Deutsche Bank Jeffrey Epstein Case
Jeffrey Epstein reaches from the grave to expose how JPMorgan profited from his sex trafficking
Epstein’s Calendar Contained Multiple Meetings With Peter Thiel
Jeffrey Epstein: Up to 130 people claim they could be child of dead financier with £470m fortune
What Is Transhumanism & Why Is Jeffrey Epstein Into It?
Hedge Funder Jeffrey Epstein and the Elton John AIDS Foundation Fight HIV Resistance
2014
2015
2016
This link will show you a PDF file of the entire list of donors who donated money to Donald Trump in the 58th presidential inaugural committee, prior to his inauguration. In page 163 of the document, Pfizer’s NYC headquarters address as well as the money they donated are on the page, with the amount being listed as exactly 1,000,000$ USD. It also lists the date from when he received it, which states it as December 22, 2016.
Donald Trump was paid by Pfizer to promote the covid-19 vaccine https://docquery.fec.gov/pdf/286/201704180300150286/201704180300150286.pdf
2018
‘Warp speed’ technology must be ‘force for good’ UN chief tells web leaders | UN News news.un.org/en/story/20…
Bill Gates has a warning about population growth
2019
Three months before "COVID," Antony Fauci and friends plan a "disruptive event"
Exclusive: The Dossier uncovers WEF-Gates-Hopkins Event 201 launch announcement from Davos 2019
https://muse.jhu.edu/pub/1/oa_edited_volume/chapter/2696549#fm9-1
Interesting that the executive order is now not more available …
Thanks to internet archive time machine we can restore the site …

By the authority vested in me as President by the Constitution and the laws of the United States of America, including section 301 of title 3, United States Code, it is hereby ordered as follows:

Official government documents show that messenger RNA (mRNA) vaccines were in development long before Donald „father of the vaccine“ Trump officially launched Operation Warp Speed on May 15, 2020 – and his daughter Ivanka admitted this in a tweet from later in the year that many seem to have missed.
A public-private partnership involving both the government and the private sector started funneling resources into the development of covid injections on Jan. 13, 2020, Ivanka Trump tweeted on Nov. 16, 2020. She wrote:
„Fact Check: This Moderna / NIH vaccine is literally the one that President @realDonaldTrump partnered with Moderna to create on January 13, 2020 … I repeat January 13th, 2020.
Just be happy. This is great news for America and for the world!“
That „great news“ ultimately led to tens of millions of vaccine injuries and deaths – and counting – all around the world. But the real kicker here is what Ivanka Trump admitted: covid vaccines were already in development before the first confirmed case of covid was even announced on Jan. 20, 2020.
„So apparently, taking Ivanka at her word, this seems to be a rather big fly in the ointment concerning the development of the mRNA vaccines; that the Trump administration was already aware of the mRNA tech, the development of the shots, and a partnership with Moderna that was already in place an entire week before the first purported case of Covid-19 was confirmed (January 20th, 2020),“ reported Wine Press News.
„However, many people did not seem to truly acknowledge and comprehend what Ivanka was admitting to, but rather blasted her and Donald for essentially knowing about the ‚threat‘ of the virus but chose not to alert people sooner, attributing hundreds of thousands of deaths to Trump’s perceived carelessness.“
How long ago did covid vaccine development REALLY begin? And how long has Donald Trump REALLY been involved?
Several large disbursements of cash were made after Jan. 20, 2020, including a $483 million grant made to Moderna on April 16 and a $456 million grant to Johnson & Johnson (J&J) on March 30. Before that, though – and before the first confirmed covid case – Donald Trump was working with Moderna to unleash mRNA jabs.
Once again, the argument that was sparked on Twitter between Ivanka Trump and her father’s „haters“ centered around the wrong issue. While they fought about who should receive credit for Operation Warp Speed, many people overlooked the fact that Donald Trump was involved in covid vaccine development before covid even emerged in the United States.
„But these tweets and more are beside the point in the much grander scheme of it all: it means these vaccines were not developed at ‚warp speed‘ like the administration says it was,“ Wine Press News further explains.
„… the Trump administration was already prepping for a ‚pandemic‘ in advance before 2020.
In 2019 Donald Trump initiated what was called ‚Crimson Contagion:‘ a pandemic simulation event identical to the likes of the infamous Event 201 simulation, that prophesied a deadly and highly infectious ‚new coronavirus‚ to sweep the globe, where many of the measures practiced in advance came to pass.“
That Crimson Contagion event culminated with Trump signing an executive order on Sept. 19, 2019 – before the very first cases of covid ever appeared anywhere in the world – allowing for new „influenza“ vaccines to be developed – so-called „flu shots“ that look oddly like covid injections.
For more details on Crimson Contagion and the subsequent actions, checkout the full report below:
SEE:
With more and more people beginning to finally take notice of the World Economic Forum’s plans, such as them touting what they call “The Great Reset,” and “You’ll own nothing and be happy” by the year 2030, along with the push for “stakeholder capitalism,” and the “4th Industrial Revolution.”
Jacinda Ardern
Vladimir Putin
Justin Trudeau
Emmanuel Macron
Angela Merkel
Bill Gates
Jeff Bezos
Tony Blair
Mark Zuckerberg
Lachlan Murdoch
Anderson Cooper
Chelsea Clinton
Nikki Haley
Gavin Newsom
Alicia Garza (founder of Black Lives Matter)
Johnathan Soros (son of George Soros)
So, this provides context into who Ivanka Trump is rubbing elbows with and is accepted into their club.
Hence, this would explain why the WEF would include her photo in a video by the Forum, explaining their Great Reset:
When the United States grows, so does the world. American prosperity has created countless jobs around the globe and the drive for excellence, creativity and innovation in the United States has led to important discoveries that help people everywhere live more prosperous and healthier lives.
States grows, so does the world. American prosperity has created countless jobs around the globe and the drive for excellence, creativity and innovation in the United States has led to important discoveries that help people everywhere live more prosperous and healthier lives.
As the United States pursues domestic reforms to unleash jobs and growth, we are also working to reform the international trading system so that it promotes broadly-shared prosperity and rewards those who play by the rules.???
We cannot have free and open trade if some countries exploit the system at the expense of others. We support free trade, but it needs to be FAIR and RECIPROCAL.???
Because in the end, unfair trade undermines us all. ???
The United States will no longer turn a blind eye to unfair economic practices, including massive intellectual property theft, industrial subsidies, and pervasive state-led economic planning. These and other predatory behaviors are distorting global markets and harming businesses and workers—not just in the United States, but around the globe. ???
Just like we expect the leaders of other countries to protect their interests, as President of the United States, I will always protect the interests of our country, our companies, and our workers.
We will enforce our trade laws and restore integrity to the trading system.
Only by insisting on FAIR and RECIPROCAL trade can we create a system that works not just for the United States but for all nations.
As I have said, the United States is prepared to negotiate mutually beneficial bilateral trade agreements with all countries. This includes the countries in TPP 11, which are very important. We have agreements with several of them already. We would consider negotiating with the rest, either individually, or perhaps as a group, if it is in all of our interests.
My administration is also taking swift action in other ways to restore American confidence and independence. We are lifting self-imposed restrictions on energy production to provide affordable power to our citizens and businesses, and to promote ENERGY SECURITY for our friends around the world. NO COUNTRY should be held hostage to a single provider of energy. ???
AMERICA IS ROARING BACK – and now is the time to INVEST in the future of America. We have dramatically cut taxes to make America competitive. We are eliminating burdensome regulations at a record pace. We are reforming the bureaucracy to make it lean, responsive and accountable—and we are ensuring our laws are enforced fairly. We have the best colleges and universities in the world, and we have the best WORKERS in the world. Energy is abundant and affordable. There has never been a better time to come to America.
We are also making historic investments in the American military—because we cannot have prosperity without security.
To make the world safer from rogue regimes, terrorism, and revisionist powers, we are asking our friends and allies to invest in their own defenses and to meet their financial obligations.
Our common security requires everyone to contribute their fair share.
My administration is proud to have led historic efforts, at the United Nations Security Council and around the world, to unite all civilized nations in our campaign of MAXIMUM PRESSURE to denuclearize the Korean peninsula. And we continue to call on partners to confront Iran’s support for terrorists and to block Iran’s path to a nuclear weapon.
We are also working with allies and partners to destroy jihadist terrorist organizations such as ISIS.
The United States is leading a broad coalition to deny terrorists control of their territory and populations, to cut off their funding, and to discredit their wicked ideology. I am pleased to report that the Coalition to Defeat ISIS has retaken almost 100 percent of the territory once held by these killers in Iraq and Syria. There is still more fighting and work to be done to consolidate our gains.
And we are committed to ensuring that Afghanistan never again becomes a safe haven for terrorists who want to commit mass murder of our citizens. ??? I want to thank those nations represented here today that have joined in these crucial efforts. You are not just securing your own citizens, but saving lives and restoring hope for millions.
The United States is leading a broad coalition to deny terrorists control of their territory and populations, to cut off their funding, and to discredit their wicked ideology. I am pleased to report that the Coalition to Defeat ISIS has retaken almost 100 percent of the territory once held by these killers in Iraq and Syria. There is still more fighting and work to be done to consolidate our gains. And we are committed to ensuring that Afghanistan never again becomes a safe haven for terrorists who want to commit mass murder of our citizens. I want to thank those nations represented here today that have joined in these crucial efforts. You are not just securing your own citizens, but saving lives and restoring hope for millions.
When it comes to terrorism, we will do what is necessary to protect our nation – we will defend our citizens, and our borders.
We are also securing our immigration system, as a matter of both national and economic security.
America is a cutting-edge economy, but our immigration system is stuck in the past.
We must replace our current system of extended-family chain migration with a merit-based system of admissions that selects new arrivals based on their ability to contribute to our economy, to support themselves financially, and to strengthen our country.
In rebuilding America, we are also fully committed to developing our workforce. We are lifting people from DEPENDENCE to INDEPENDENCE – because we know the single best anti-poverty program is a PAYCHECK.
To be successful, it is not enough to invest in our economy – we must invest in OUR PEOPLE.
When people are forgotten, the world becomes fractured. Only by hearing and responding to the voices of the forgotten can we create a bright future that is truly shared by all.
A nation’s greatness is more than the sum of its production – a nation’s greatness is the sum of its citizens: The values, pride, love, devotion and character of the people who call that nation home.
From my first international G-7 summit, to the G-20, to the U.N. General Assembly, to APEC, to the World Trade Organization, and today at the World Economic Forum, my administration has not only been present, but has driven our message that we are all stronger when free and sovereign Nations cooperate toward shared goals—and shared dreams.
Represented in this room are some remarkable citizens from all over the world—you are national leaders, business titans, industry giants, and many of the brightest minds in many fields.
Each of you has the power to change hearts, transform lives, and shape your countries’ destinies. With this power, comes an obligation—a duty of loyalty to the people, workers, and customers who have made you who you are.
So together, let us resolve to use our power, our resources, and our voices, not just for ourselves, but for our people—to lift their burdens, to raise their hopes, and to empower their dreams. To protect their families, their communities, their histories, and their futures.
That’s what we’re doing in America, and the results are unmistakable. It’s why new businesses and investment are flooding in. It’s why our unemployment rate is the lowest it’s been in decades. It’s why America’s future has never been brighter.
Today, I am inviting all of you to become part of this incredible future we are building together.
Thank you to our hosts, thank you to the leaders and innovators in the audience, but most importantly, thank you to all the hardworking men and women who do their duty each and every day – making this a better world for everyone. Together, let us send our love and gratitude to them—because they make our countries run.
Thank you, God Bless You, and God Bless you all!
2020 Operation Warp Speed
A $14 billion investment
The extraordinary vaccine initiative was propelled by a $14 billion investment from the federal government — which Trump supported and signed off on — that would become Operation Warp Speed, a public-private partnership to hasten vaccines and treatments, in part by footing the bill to manufacture millions of doses before anyone knew if they worked.
‘A 15-year, overnight success story’
The public-private partnership created through Operation Warp Speed is not an idea the administration invented. For years, global public health leaders had talked about using government investment to reduce the financial risks that dissuade companies from developing needed products. That was also the model behind the creation of the Biomedical Advanced Research and Development Authority (BARDA) during the George W. Bush administration, with an eye toward working with private industry on bioterrorism countermeasures.
‘I heard everybody explaining … why it will never work’
In April, after Marks and Kadlec gave Azar their proposal, the secretary presented it to the White House coronavirus task force. Some were skeptical about whether it would work and wary of the enormous price tag.
A bruising year for the FDA turns more tumultuous
After helping to launch Warp Speed, Marks quickly retreated to the FDA. He and Slaoui had clashed, according to individuals familiar with the situation who spoke on the condition of anonymity because they were not authorized to discuss the issue. Marks also realized he was most valuable to the vaccine effort
How „Operation Warp Speed’s“ Big Vaccine Contracts Could Stay Secret
President Trump announced the creation of Operation Warp Speed in May to fast-track a coronavirus vaccine. He called it "a massive scientific and industrial, logistic endeavor unlike anything our country has seen since the Manhattan Project."
How the ‘deep state’ scientists vilified by Trump helped him deliver an unprecedented achievement
A $14 billion partnership between government and industry is spurring the quickest vaccine development in U.S. history
Ivanka Trump Admits In Tweet That The Trump Admin. Already Partnered With Moderna To Produce MRNA Vaccines BEFORE Operation Warp Speed
January 13, 2023
So apparently, taking Ivanka at her word, this seems to be a rather big fly in the ointment concerning the development of the mRNA vaccines; that the Trump administration was already aware of the mRNA tech, the development of the shots, and a partnership with Moderna that was already in place an entire week before the first purported case of Covid-19 was confirmed (January 20th, 2020).
The Sabin-Aspen Vaccine Science & Policy Group - The Aspen Institute
Beyond COVID-19
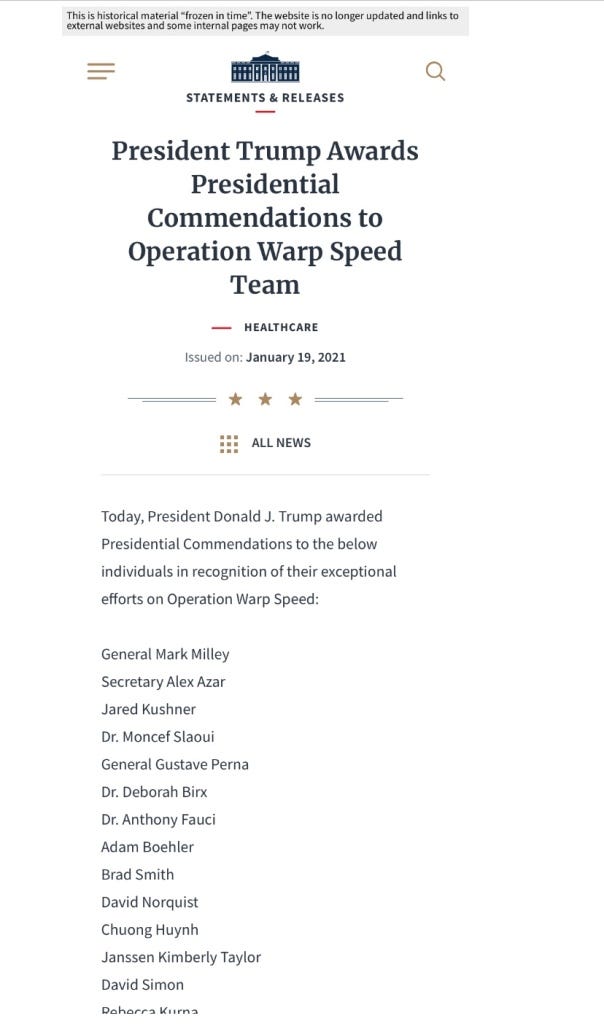
Public perceptions of health and social care in light of COVID-19 (November 2020)
The Truth About COVID-19’s Deadliness:
Covid-19: Researchers face wait for patient level data from Pfizer and Moderna vaccine trials
Executive Summary for Litigation of PANDEMIC Crimes…
The ‘very, very bad look' of remdesivir, the first FDA-approved COVID-19 drug
The Food and Drug Administration held no advisory meeting on antiviral, and the European Union signed contract without knowing of failed trial
https://x.com/DrDMartinWorld/status/1659648084488355840?s=20
CEPI launches 100-day vaccine “moonshot”
Ann Danaiya Usher
Published:March 19, 2022DOI:
https://doi.org/10.1016/S0140-6736(22)00513-X
Drug hailed by Hancock 'has no benefit'
A drug that Matt Hancock described as the "biggest step forward in the treatment of coronavirus since the crisis began" has failed to show any benefit in randomised controlled trials.
It wasn't just the UK where remdesivir was hailed - the US bought up almost the entire global supply from US pharmaceutical giant Gilead Sciences. It was approved for use there.
The drug was developed to treat Ebola.
In five European countries, researchers studied 843 COVID-19 patients who were hospitalised between March 2020 and January 2021 and who needed oxygen or machines to help with breathing.
Two weeks after patients had received either the antiviral remdesivir plus standard of care, or standard of care alone, for up to 10 days, there was no difference between the groups in signs of improvement.
Remdesivir not only does not have any benefit, but it has a horrific safety profile.
Conclusions: Deterioration of liver and kidney function are frequently observed ADEs with remdesivir
Of course, the infamous Ebola trial that showed the true deadly profile of this drug:
“However, six months into the Ebola study, the trial’s Safety Review Board suddenly pulled both remdesivir and ZMapp from the trial. 12 Remdesivir, it turned out, was hideously dangerous. Within 28 days, subjects taking Remdesivir had lethal side effects including multiple organ failure, acute kidney failure, septic shock, and hypotension, and 54 percent of the remdesivir group died—the highest mortality rate among the four experimental drugs. 13”
Combine renal failure with intubation and that is what padded the “excess deaths” of the Covid-19 “pandemic.”
Therefore, when the MSM comes out with claims that Remdesivir has no benefit, what they are really doing is engaging in a coverup. BigPharma, FDA, CDC, WHO, NIH, Pentagon, et al. all knew the true “safety” profile of this deadly drug.
Women are much more likely than men to have an array of so-called autoimmune diseases, like lupus and multiple sclerosis. A new study offers an explanation rooted in the X chromosome.
DNA fragments from thousands of years ago are providing insights into multiple sclerosis, diabetes, schizophrenia and other illnesses. Is this the future of medicine?
A study of DNA from half a million volunteers found hundreds of mutations that could boost a young person’s fertility and that were linked to bodily damage later in life.
In the first effort of its kind, researchers now have linked DNA from 27 African Americans buried in the cemetery to nearly 42,000 living relatives.
Environmental DNA research has aided conservation, but scientists say its ability to glean information about humans poses dangers.
That person who looks just like you is not your twin. But if scientists compared your genomes, they might find a lot in common.
Industry Describing Product-Specific Bioequivalence
How Palantir is strengthening its hold on NHS patient data
Coronavirus Gives a Dangerous Boost to DARPA’s Darkest Agenda
„Exploring the Oxford-AstraZeneca Eugenics Links“ (2021)
In its report, A Global Deal for Our Pandemic Age, the G20 High Level Independent Panel on Financing the Global Commons for Pandemic Preparedness and Response (the Panel) finds that the world remains very poorly equipped to prevent or contain future epidemics or pandemics. The Panel calls on the G20 and international community to move swiftly to close current shortfalls in the international COVID-19 response. However, the world cannot wait for COVID-19 to be over to make the global investments and reforms that are critically needed to head off future pandemics, which threaten to be more frequent and increasingly dangerous.
Lawmakers and policymakers place great value in precedent, whether written or historical. There is almost no precedent to guide FDA’s use of this relatively new power, let alone during the greatest public health crisis the agency has ever faced. On the contrary, the history of EUAs points up essential dilemmas that aren’t going away, and that will have to be grappled with in emergencies to come.
These questions are not unique to FDA. Much has been written generally about the role of agency norms and institutional dynamics in the administrative state. Others have discussed the myriad violations of norms of democratic governance by the Trump administration (arguably the defining legacy of the Trump years). These issues cannot be resolved by looking to the law; they will inevitably require hard judgments about how to balance deference to scientific expertise with public accountability, how to integrate empirical analysis and value judgments, and how to weigh our competing values in times of crisis.
FDA’s emergency powers underscore the impact these questions have on the public welfare — and demonstrate that the way we answer them can literally be a matter of life and death.
The ideology of lockdown is a menace to society
Lessons Learned from Covid Deaths in Massachusetts with John Beaudoin Sr.
AARON SIRI GIVES TESTIMONY TO THE ARIZONA STATE SENATE
MSU technology transfer infrastructure and personnel, and with the CEPI program to develop partnerships with vaccine manufacturers
Regionalized Vaccine Manufacturing Collaborative A Framework for Enhancing Vaccine Access Through Regionalized Manufacturing Ecosystems
INSIGHT REPORT JANUARY 2024
https://www3.weforum.org/docs/WEF_Regionalized_Vaccine_Manufacturing_Collaborative_2024.pdf
Related
Benefits and Drawbacks of Emergency Use Authorizations for COVID Vaccines
By Sravya Chary
Two COVID-19 vaccine manufacturers recently submitted Emergency Use Authorization (EUA) requests to the Food and Drug Administration (FDA) for their candidates.
While the need for a safe and efficacious COVID-19 vaccine is dire and immediate, an EUA may not be the best regulatory method to provide access. Experts warn that the EUA pathway may impede vital scientific progress needed to establish the long term safety and efficacy of investigational COVID-19 vaccines.
According to the FDA, an Emergency Use Authorization is a tool that allows an unapproved medical product to be released to the public in a health crisis given that the medical product meets statutory criteria outlined in Section 564 of the Federal Food, Drug, and Cosmetic Act.
The statutory criteria are as follows: 1) an emergency must be declared, 2) the agent specified for the declaration of the emergency can cause a serious or life-threatening disease/condition, 3) it is reasonable to believe the medical product is effective in treating, diagnosing, or preventing that disease/condition based on scientific evidence, 4) the known potential benefits outweigh the known potential risks, and 5) there is no adequate, approved, alternate treatment available.
Although the issuance of an EUA is beneficial in that it would allow the public to access a potentially promising vaccine without having to wait through the long FDA approval process, experts have stated concerns regarding the impediment an EUA may pose to accurate assessments of COVID-19 vaccines for safety and efficacy.
In October, the FDA issued a guidance document for vaccine manufacturers with information regarding the data they must provide in order for the agency to issue an EUA.
On November 18, 2020, Pfizer and BioNTech released a statement that following the conclusion of a phase 3 study of their COVID-19 vaccine candidate, in which all primary efficacy endpoints were met, the organization planned to submit an EUA request to the FDA.
Moderna recently followed suit as the second vaccine developer to apply for an EUA.
But experts have expressed concern with issuing an EUA for these COVID-19 vaccines.
David Cyranoski writes in Nature that for vaccine trials, initially participants are blinded, and therefore unaware of whether they receive the vaccine or a placebo. However, following the issuance of an EUA, the company would have an ethical obligation to disclose the vaccine as an option to those trial participants in the placebo arm. This raises scientific concern. If too many individuals in the placebo arm cross over, the control group may not be large enough to be used as a comparator. Statistically significant results may be too difficult to gather, rendering the results ineffective for drawing conclusions on the long term safety and efficacy of the vaccine.
Just weeks prior to receiving requests for EUAs, the FDA has shown signs of “getting cold feet” over the issuance of an EUA for a COVID-19 vaccine.
According to Arnold Monto, acting chair of the FDA’s Vaccines and Related Biological Products Advisory Committee and leading epidemiologist at the University of Michigan’s School of Public Health, vaccine developers may not be able to generate enough data to “successfully apply for a full license” if issued an EUA.
Further Stephanie Schrag, an epidemiologist at the Centers for Disease Control and Prevention (CDC), warns that an EUA may impede the collection of information regarding secondary endpoints, such as whether the vaccine decreases the number of severe COVID-19 cases, and its efficacy in those disproportionately affected (the elderly and people of color!?).
Another avenue that has been raised as a potential regulatory pathway is expanded access. Expanded access, otherwise known as compassionate use, is a pathway through which a patient can gain access to the investigational vaccine outside of clinical trials. The expanded access route would allow those who are at highest risk to access the investigational vaccine without “opening the floodgates” in the way an EUA would. According to Jesse Goodman, a former FDA scientist, expanded access could ensure the continuation of clinical trials, however, the expanded access process is inefficient and strenuous.
The FDA will meet to evaluate the Pfizer application on December 10 and Moderna’s on December 17. Although an EUA is likely in the cards for at least one of these investigational vaccines, an issuance may eventually prove to be disastrous for our understanding of overall safety and efficacy.
The above opinions are wholly my own and in no way represent the opinions of my associated institutions.
What’s the Difference Between Vaccine Approval (BLA) and Authorization (EUA)?
Authorize Emergency Vaccines for COVID-19, but Do It Well
Patent trail
1993 – Wo1993023422A1 = methods vaxing Corona Felines #SmithKline Pharna
2002 – Wo2002086068A2 = SARS CoV 1 UNC Health USA
2005 – Wo2005049814A2 = Pneumonia Causing Virus France
2005 – Wo2005060520A3 = antibodies Dana-Farber Cancer Institute
2005 – WO2005081716A2 = DNA SARS John Hopkins University
2009 – Wo2009051837A3 = Vax nanotech #MIT & #Harvard
2015 – Wo2015143335A1 = Chimeric Spike Protein UNC Health, University of Chapel Hill, Ralph BARIC
2017 – Wo2017044507A2 = MERS-CoV Nanoparticle therapeutics
Wo2017049245A2 = Intracellular Triple Helix Moderna
Wo2020060606A1 = Crypto mining people @ $MSFT
Wo2022034572A1 = POLICY prioritizing jabs Israel
David E. Martin proved that since 1999, 73 patents back that SARS-CoV-2 is not a wild novel virus but a weaponized chimeric lab-created virus.
The Emergency Use Authorization (EUA) authority allows FDA to help strengthen the nation’s public health protections against CBRN threats by facilitating the availability and use of MCMs needed during public health emergencies.
Under section 564 of the Federal Food, Drug, and Cosmetic Act (FD&C Act), the FDA Commissioner may allow unapproved medical products or unapproved uses of approved medical products to be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions caused by CBRN threat agents when there are no adequate, approved, and available alternatives.
Section 564 of the FD&C Act was amended by the Project Bioshield Act of 2004 and the Pandemic and All-Hazards Preparedness Reauthorization Act of 2013 (PAHPRA), which was enacted in March 2013
Guidance
In January 2017, FDA finalized the guidance: Emergency Use Authorization of Medical Products and Related Authorities. For more information, please see the January 13, 2017 Federal Register notice.
Questions & Answers
In January 2014, FDA issued a question and answer document (PDF, 762K) to respond to questions raised by public health stakeholders about PAHPRA’s amendments to the EUA authority and establishment of new authorities related to the emergency use of MCMs during CBRN emergencies.
Current EUAs
The tables below provide information on current EUAs:
Anthrax: Doxycycline Mass Dispensing EUA Information and National Postal Model Anthrax EUA Information
Middle East Respiratory Syndrome Coronavirus (MERS-CoV) EUA Information
Information about EUAs that are no longer in effect is available on our EUA archive page.
On December 9, 2015, the HHS Secretary amended seven Public Readiness and Emergency Preparedness (PREP) Actdeclarations, continuing PREP Act coverage for pandemic influenza, Ebola virus disease vaccines and therapeutics, anthrax, botulinum toxin, smallpox, and Acute Radiation Syndrome medical countermeasures. On December 12, 2016, the HHS Secretary amended two PREP Act declarations, continuing PREP Act coverage for Ebola virus disease vaccines and therapeutics. Also see PREP Act FAQs.
Anthrax: Doxycycline Mass Dispensing EUA Information*
*Note: Based on the April 13, 2016, issuance of the Doxycycline Emergency Dispensing Order and Doxycycline Emergency Use Instructions (EUI), which replace the need for an EUA for doxycycline mass dispensing, this EUA will be terminated by FDA. Notice of such termination will be published in the Federal Register.
More: current drug information related to anthrax, from the Center for Drug Evaluation and Research
Medical Product
Oral formulations of doxycycline products for the post-exposure prophylaxis (PEP) of inhalational anthrax
Date of EUA Issuance
July 27, 2011
Letter of Authorization
2011 Letter of Authorization(PDF, 9.0 MB)
Federal Register Notice for EUA
Authorization of Emergency Use of Oral Formulations of Doxycycline (August 4, 2011)
Fact Sheets and Labeling
Healthcare(PDF, 140 KB)
Patients (Recipients)(PDF, 137 KB)
Doxycycline – Home Preparation Instructions for Children or Adults Who Cannot Swallow Pills(PDF, 189 KB)
In an Emergency: How to Prepare Doxycycline for Children or Adults Who Cannot Swallow Pills(PDF, 1 MB)
EUA Determination and Declaration (Effective Date)
Renewal of Declaration Regarding Emergency Use of All Oral Formulations of Doxycycline Accompanied by Emergency Use Information (July 20, 2012)
Renewal of Declaration on Emergency Use of Doxycycline (July 20, 2011)
PREP Act Declaration (if applicable)
Anthrax Medical Countermeasures-Amendment (December 9, 2015)
Anthrax: National Postal Model Anthrax EUA Information
Medical ProductDate of EUA IssuanceLetter of AuthorizationFederal Register Notice for EUA Fact Sheets and LabelingEUA Determination and Declaration (Effective Date)PREP Act Declaration (if applicable)
Oral formulations of doxycycline products for the post-exposure prophylaxis (PEP) of inhalational anthrax
October 14, 2011
(originally issued on October 6, 2008, with subsequent amendments)
Amendment to the Letter of Authorization (October 14, 2011)(PDF, 7 MB)
Amendment to the Letter of Authorization (August 23, 2010)(PDF, 2 MB)
Amendment to the Letter of Authorization (February 25, 2009) (PDF, 97 KB)
Renewal of Declaration ... (July 20, 2012)
Renewal of Declaration ... (July 20, 2011)
Renewal of Declaration ... (October 1, 2010)
Renewal of Declaration ... (October 1, 2009)
Determination and Declaration Regarding Emergency Use of Doxycycline Hyclate Tablets Accompanied by Emergency Use Information – Federal Register (October 1, 2008)
Anthrax Medical Countermeasures-Amendment (December 9, 2015)
Ebola Virus EUA Information
Additional information on 2014-2015 Ebola virus EUAs (diagnostics)
Ebola response updates from FDA (all agency activities)
Medical ProductDate of EUA IssuanceLetter of AuthorizationFederal Register Notice for EUAFact Sheets and Labeling EUA Determination and Declaration (Effective Date)PREP Act Declaration (if applicable)EZ1 Real-time RT-PCR Assay
(DoD)
August 5, 2014 (initial issuance)
October 10, 2014 (reissuance)
Authorization(PDF, 61 KB) FR notice
Healthcare(PDF, 58 KB)
Patients(PDF, 59 KB)
Labeling (PDF, 1.1 MB)
Declaration Regarding Emergency Use of In Vitro Diagnostics for Detection of Ebola Virus (August 4, 2014) CDC Ebola Virus NP Real-time RT-PCR Assay
(CDC)
October 10, 2014 (initial issuance)
March 2, 2015 (reissuance)
Authorization(PDF, 282 KB) FR notice
Healthcare(PDF, 207 KB)
Patients(PDF, 149 KB)
Labeling (PDF, 496 KB)
Declaration Regarding Emergency Use of In Vitro Diagnostics for Detection of Ebola Virus (August 4, 2014)
CDC Ebola Virus VP40 Real-time RT-PCR Assay
(CDC)
October 10, 2014 (initial issuance)
March 2, 2015 (reissuance)
Authorization(PDF, 285 KB) FR notice
Healthcare(PDF, 207 KB)
Patients(PDF, 149 KB)
Labeling (PDF, 494 KB)
Declaration Regarding Emergency Use of In Vitro Diagnostics for Detection of Ebola Virus (August 4, 2014)
FilmArray NGDS BT-E Assay
(Biofire Defense, LLC)
October 25, 2014 (initial issuance)
March 2, 2015 (reissuance)
Authorization(PDF, 326 KB) FR notice
Healthcare(PDF, 40 KB)
Patients(PDF, 40 KB)
Instructions for Use (PDF, 740 KB)
Declaration Regarding Emergency Use of In Vitro Diagnostics for Detection of Ebola Virus (August 4, 2014)
FilmArray Biothreat-E test
(Biofire Defense, LLC)
October 25, 2014
Authorization(PDF, 73 KB) FR notice
Healthcare(PDF, 46 KB)
Patients(PDF, 40 KB)
Instructions for Use (PDF, 1.2 MB)
Declaration Regarding Emergency Use of In Vitro Diagnostics for Detection of Ebola Virus (August 4, 2014)
RealStar® Ebolavirus RT-PCR Kit 1.0
(altona Diagnostics, GmbH)
November 10, 2014 (initial issuance)
November 26, 2014 (reissuance)
Authorization(PDF, 263 KB) FR notice
Healthcare(PDF, 81 KB)
Patients(PDF, 92 KB)
Instructions for Use (PDF, 634 KB)
Declaration Regarding Emergency Use of In Vitro Diagnostics for Detection of Ebola Virus (August 4, 2014)
LightMix® Ebola Zaire rRT-PCR Test
(Roche Molecular Systems, Inc.)
December 23, 2014
Authorization(PDF, 2.2 MB) FR notice
Healthcare(PDF, 59 KB)
Patients(PDF, 60 KB)
Instructions for Use (PDF, 328 KB)
Declaration Regarding Emergency Use of In Vitro Diagnostics for Detection of Ebola Virus (August 4, 2014)
ReEBOV™ Antigen Rapid Test
(Zalgen Labs, LLC)
February 24, 2015 (initial issuance)
March 16, 2015 (reissuance)
November 3, 2016 (reissuance)
Authorization(PDF, 54 KB) FR notice
Healthcare(PDF, 59 KB)
Patients (PDF, 95 KB)
Instructions for Use (PDF, 328 KB)
Declaration Regarding Emergency Use of In Vitro Diagnostics for Detection of Ebola Virus (August 4, 2014)
Xpert® Ebola Assay
(Cepheid)
March 23, 2015
Authorization(PDF, 240 KB) FR notice
Healthcare(PDF, 310 KB)
Patients(PDF, 211 KB)
Instructions for Use (PDF, 625 KB)
Declaration Regarding Emergency Use of In Vitro Diagnostics for Detection of Ebola Virus (August 4, 2014)
OraQuick® Ebola Rapid Antigen Test - for use with whole blood
(OraSure Technologies, Inc.)
July 31, 2015
Authorization(PDF, 250 KB) FR notice
Healthcare(PDF, 109 KB)
Patients(PDF, 93 KB)
Labeling (PDF, 1.3 MB)
Declaration Regarding Emergency Use of In Vitro Diagnostics for Detection of Ebola Virus (August 4, 2014)
OraQuick® Ebola Rapid Antigen Test - for use with cadaveric oral fluid
(OraSure Technologies, Inc.)
March 4, 2016 (initial issuance)
September 26, 2016 (reissuance)
Authorization(PDF, 266 KB) FR notice
Response team(PDF, 120 KB)
Relatives and caregivers(PDF, 96 KB)
Labeling (PDF, 253 KB)
Declaration Regarding Emergency Use of In Vitro Diagnostics for Detection of Ebola Virus (August 4, 2014)
Idylla™ Ebola Virus Triage Test (Biocartis NV)
May 26, 2016
Authorization (PDF, 321 KB) FR notice
Fact Sheet for Healthcare Practitioner (PDF, 203 KB)
Fact Sheet for Patients (PDF, 163KB)
Instructions for Use (PDF, 2.12MB)
Declaration Regarding Emergency Use of In Vitro Diagnostics for Detection of Ebola Virus (August 4, 2014)
Enterovirus D68 (EV-D68) EUA Information
Medical ProductDate of EUA IssuanceLetter of AuthorizationFederal Register Notice for EUAFact Sheets and Labeling EUA Determination and Declaration (Effective Date)PREP Act Declaration (if applicable)CDC Enterovirus D68 2014 Real-time RT-PCR Assay (EV-D68 2014 rRT-PCR)
May 12, 2015
Authorization(PDF, 229 KB) FR notice
Healthcare(PDF, 214 KB)
Patients(PDF, 150 KB)
Labeling(PDF, 531 KB)
Determination and Declaration Regarding Emergency Use of New In VitroDiagnostics for Detection of Enterovirus D68(February 6, 2015)
H7N9 Influenza EUA Information
Medical ProductDate of EUA IssuanceLetter of AuthorizationFederal Register Notice for EUAFact Sheet and Labeling EUA Determination and Declaration (Effective Date)PREP Act Declaration (if applicable)
CDC Human Influenza Virus Real-Time RT-PCR Diagnostic Panel-Influenza A/H7 (Eurasian Lineage) Assay
April 22, 2013Authorization(PDF, 78 KB)FR notice
Healthcare(PDF, 46 KB)
Patients(PDF, 32 KB)
Determination and Declaration Regarding Emergency Use of in Vitro Diagnostics for Detection of the Avian Influenza A (H7N9) Virus(April 19, 2013)
Additional information from HHS
Pandemic Influenza Medical Countermeasures-Amendment (December 9, 2015)Quidel Lyra™ Influenza A Subtype H7N9 AssayFebruary 14, 2014Authorization(PDF, 57 KB)FR notice
Healthcare(PDF, 42 KB)
Patients(PDF, 40 KB)
Determination and Declaration Regarding Emergency Use of in Vitro Diagnostics for Detection of the Avian Influenza A (H7N9) Virus(April 19, 2013)
Additional information from HHS
Pandemic Influenza Medical Countermeasures-Amendment (December 9, 2015)A/H7N9 Influenza Rapid TestApril 25, 2014AuthorizationFR notice
Determination and Declaration Regarding Emergency Use of in Vitro Diagnostics for Detection of the Avian Influenza A (H7N9) Virus(April 19, 2013)
Additional information from HHS
Pandemic Influenza Medical Countermeasures-Amendment (December 9, 2015)
Middle East Respiratory Syndrome Coronavirus (MERS-CoV) EUA Information
Medical ProductDate of EUA IssuanceLetter of AuthorizationFederal Register Notice for EUAFact Sheets and LabelingEUA Determination and Declaration (Effective Date)PREP Act Declaration (if applicable)CDC Novel Coronavirus 2012 Real-time RT-PCR Assay
June 5, 2013 (initial issuance)
June 10, 2014 (reissuance)
Authorization(PDF, 2.2 MB)FR notice
Contacts(PDF, 1.2 MB)
Instructions for Use(PDF, 743 KB)
Determination and Declaration Regarding Emergency Use of In Vitro Diagnostics for Detection of Middle East Respiratory Syndrome Coronavirus (MERS-CoV)(May 29, 2013)
Additional information from HHS
RealStar® MERS-CoV RT-PCR Kit U.S.
July 17, 2015
(initial issuance)
February 12, 2016 (reissuance)
Authorization(PDF, 238 KB) FR notice
Healthcare(PDF, 269 KB)
Patients(PDF, 241 KB)
Instructions for Use (PDF, 840 KB)
Fact Sheet for Asymptomatic Individuals Suspected of Exposure to MERS-CoV Cases(PDF, 285 KB)
Determination and Declaration Regarding Emergency Use of In Vitro Diagnostics for Detection of Middle East Respiratory Syndrome Coronavirus (MERS-CoV)(May 29, 2013)
Additional information from HHS
Nerve Agent EUA Information
Medical ProductDate of EUA IssuanceLetter of AuthorizationFederal Register Notice for EUAFact Sheets and LabelingEUA Determination and Declaration (Effective Date)PREP Act Declaration (if applicable)2 mg Atropine Auto-Injector (Rafa Laboratories Ltd.)
April 11, 2017 (initial issuance)
Amended May 23, 2017
Letter of Authorization(PDF, 514 KB)
Letter granting EUA amendment(s)(PDF, 28 KB)
Healthcare(PDF, 527 KB)
Patients and Caregivers(PDF, 390 KB)
Determination and Declaration Regarding Nerve Agent or Certain Insecticide (Organophosphorus and/or Carbamate) Poisoning (April 11, 2017)
Nerve Agents and Certain Insecticides (Organophosphorus and/or Carbamate) Countermeasures(April 11, 2017)
Zika Virus EUA Information
Zika virus response updates from FDA
Zika virus diagnostic development information
Draft EUA review templates for Zika are available by email request to: CDRH-ZIKA-Templates@fda.hhs.gov
Laboratory personnel using Zika diagnostic assays under EUA are encouraged to report performance concerns directly to FDA at CDRH-EUA-Reporting@fda.hhs.gov, in addition to reporting concerns to the manufacturer.
Medical ProductDate of EUA IssuanceLettersFederal Register Notice for EUA
Fact Sheets and Labeling
EUA Determination and Declaration (Effective Date)PREP Act Declaration (if applicable)
CDC Zika Immunoglobulin M (IgM) Antibody Capture Enzyme-Linked Immunosorbent Assay
February 26, 2016 (initial issuance)
Amended June 29, 2016
Amended November 15, 2016
Amended December 6, 2016
Amended May 3, 2017
Authorization(PDF, 494 KB)
Letter granting EUA amendment(s) (PDF, 532KB)
Letter granting EUA amendment(s) (PDF, 124 KB)
Letter granting EUA amendment(s) (PDF, 110 KB)
Healthcare(PDF, 74 KB)
Patients(PDF, 48 KB)
Labeling(PDF, 4.7 MB)
Emergency Use of In Vitro Diagnostic Tests for Detection of Zika Virus and/or Diagnosis of Zika Virus Infection(February 26, 2016)
CDC Trioplex Real-time RT-PCR Assay (Trioplex rRT-PCR)
March 17, 2016 (initial issuance)
Amended September 21, 2016
Amended January 12, 2017
Amended March 1, 2017
Amended April 6, 2017
Authorization(PDF, 82 KB)
Letter granting EUA amendment(s) (PDF, 223 KB)
Letter granting EUA amendment(s) (PDF, 223 KB)
Letter granting EUA amendment(s) (PDF, 223 KB)
Letter granting EUA amendment(s)(PDF, 126 KB)
Healthcare(PDF, 56 KB)
Patients(PDF, 42 KB)
Labeling(PDF, 1.3 MB)
Emergency Use of In Vitro Diagnostic Tests for Detection of Zika Virus and/or Diagnosis of Zika Virus Infection(February 26, 2016)
Zika Virus RNA Qualitative Real-Time RT-PCR (Quest Diagnostics Infectious Disease, Inc.)
April 28, 2016 (initial issuance)
Amended October 7, 2016
Amended April 11, 2017
Authorization(PDF, 339 KB)
Letter granting EUA amendment(s) (PDF, 223 KB)
Healthcare(PDF, 53 KB)
Patients(PDF, 27 KB)
Labeling(PDF, 439 KB)
Emergency Use of In Vitro Diagnostic Tests for Detection of Zika Virus and/or Diagnosis of Zika Virus Infection(February 26, 2016)
RealStar Zika Virus RT-PCR Kit U.S. (altona Diagnostics GmbH)
May 13, 2016 (initial issuance)
Amended October 31, 2016
Amended March 6, 2017
Authorization(PDF, 342 KB)
Letter Granting EUA Amendment(s) (PDF, 130 KB)
Letter Granting EUA Amendment(s) (PDF, 130 KB)
Healthcare (PDF, 474 KB)
Patients(PDF, 238 KB)
Labeling (PDF, 698 KB)
Emergency Use of In Vitro Diagnostic Tests for Detection of Zika Virus and/or Diagnosis of Zika Virus Infection(February 26, 2016)
Aptima Zika Virus assay(Hologic, Inc.)
June 17, 2016 (initial issuance)
Amended September 7, 2016
Amended April 12, 2017
Authorization(PDF, 305 KB)
Letter granting EUA amendment(s) (PDF, 126 KB)
Letter granting EUA amendment(s) (PDF, 124 KB)
Healthcare(PDF, 299 KB)
Patients(PDF, 185 KB)
Labeling(PDF, 409 KB)
Emergency Use of In Vitro Diagnostic Tests for Detection of Zika Virus and/or Diagnosis of Zika Virus Infection(February 26, 2016)
Zika Virus Real-time RT-PCR Test (Viracor Eurofins)
July 19, 2016 (initial issuance)
Amended February 28, 2017
Authorization(PDF, 334 KB)
Letter granting EUA amendment(s) (PDF, 124 KB)
Healthcare(PDF, 328 KB)
Patients(PDF, 154 KB)
Labeling(PDF, 631 KB)
Emergency Use of In Vitro Diagnostic Tests for Detection of Zika Virus and/or Diagnosis of Zika Virus Infection(February 26, 2016)
VERSANT® Zika RNA 1.0 Assay (kPCR) Kit (Siemens Healthcare Diagnostics Inc.)
July 29, 2016 (initial issuance)
Amended December 19, 2016
Authorization(PDF, 78 KB)
Letter granting EUA amendment(s) (PDF, 124 KB)
Healthcare(PDF, 170 KB)
Patients(PDF, 122 KB)
Labeling(PDF, 511 KB)
Emergency Use of In Vitro Diagnostic Tests for Detection of Zika Virus and/or Diagnosis of Zika Virus Infection(February 26, 2016)
xMAP® MultiFLEX™ Zika RNA Assay(Luminex Corporation)
August 4, 2016 (initial issuance)
Amended January 7, 2017
Amended May 19, 2017
Authorization(PDF, 505 KB)
Letter granting EUA amendment(s) (PDF, 126 KB)
Letter granting EUA amendment(s) (PDF, 126 KB)
Healthcare (PDF, 258 KB)
Patients(PDF, 157 KB)
Labeling(PDF, 701 KB)
Emergency Use of In Vitro Diagnostic Tests for Detection of Zika Virus and/or Diagnosis of Zika Virus Infection(February 26, 2016)
ZIKV Detect™ IgM Capture ELISA (InBios International, Inc.)
August 17, 2016 (initial issuance)
Amended March 27, 2017
Authorization(PDF, 528 KB)
Letter granting EUA amendment(s) (PDF, 125 KB)
Healthcare(PDF, 280 KB)
Patients(PDF, 301 KB)
Labeling(PDF, 567 KB)
Emergency Use of In Vitro Diagnostic Tests for Detection of Zika Virus and/or Diagnosis of Zika Virus Infection(February 26, 2016)
Sentosa® SA ZIKV RT-PCR Test (Vela Diagnostics USA, Inc.)
September 23, 2016
Authorization(PDF, 355 KB) FR notice
Healthcare(PDF, 265 KB)
Patients(PDF, 236 KB)
Labeling(PDF, 1,987 KB)
Emergency Use of In Vitro Diagnostic Tests for Detection of Zika Virus and/or Diagnosis of Zika Virus Infection(February 26, 2016)
Zika Virus Detection by RT-PCR Test(ARUP Laboratories)
September 28, 2016
Authorization(PDF, 98 KB) FR notice
Healthcare(PDF, 52 KB)
Patients(PDF, 200 KB)
Labeling(PDF, 501 KB)
Emergency Use of In Vitro Diagnostic Tests for Detection of Zika Virus and/or Diagnosis of Zika Virus Infection(February 26, 2016) Abbott RealTime ZIKA(Abbott Molecular Inc.)
November 21, 2016 (initial issuance)
Amended January 6, 2017
Authorization(PDF, 84 KB)
Letter granting EUA amendment(s) (PDF, 150 KB)
Healthcare(PDF, 203 KB)
Patients(PDF, 217 KB)
Labeling(PDF, 1189 KB)
Emergency Use of In Vitro Diagnostic Tests for Detection of Zika Virus and/or Diagnosis of Zika Virus Infection(February 26, 2016) Zika ELITe MGB® Kit U.S.(ELITechGroup Inc. Molecular Diagnostics)December 9, 2016Authorization(PDF, 312 KB) FR notice
Healthcare(PDF, 213 KB)
Patients(PDF, 179 KB)
Labeling (PDF, 718 KB)
Emergency Use of In Vitro Diagnostic Tests for Detection of Zika Virus and/or Diagnosis of Zika Virus Infection(February 26, 2016) Gene-RADAR® Zika Virus Test(Nanobiosym Diagnostics, Inc.)March 20, 2017Authorization(PDF, 313 KB)
Healthcare(PDF, 267 KB)
Patients(PDF, 240 KB)
Labeling(PDF, 338 KB)
Emergency Use of In Vitro Diagnostic Tests for Detection of Zika Virus and/or Diagnosis of Zika Virus Infection(February 26, 2016) LIAISON® XL Zika Capture IgM Assay (DiaSorin Incorporated)April 5, 2017Authorization(PDF, 363 KB)
Healthcare(PDF, 253 KB)
Patients(PDF, 235 KB)
Labeling(PDF, 214 KB)
Emergency Use of In Vitro Diagnostic Tests for Detection of Zika Virus and/or Diagnosis of Zika Virus Infection(February 26, 2016)
Related Links
Pandemic and All-Hazards Preparedness Reauthorization Act of 2013 (PAHPRA)
Historical Information about Device Emergency Use Authorizations
MCM-Related Cooperative Arrangements
FDA and the Medical Countermeasures Initiative use a variety of cooperative arrangements to further development and availability of medical countermeasures (MCMs). These include Memoranda of Understanding and Confidentiality Commitments. Current arrangements are listed below by category, then by effective date, with the newest first in each list.
Memoranda of Understanding (MOUs)
FDA and the Defense Advanced Research Projects Agency (DARPA) [MOU 225-17-015]
June 1, 2017: Promotes collaboration between FDA and DARPA, and provides a mechanism for the sharing of certain nonpublic information (Replaces MOU 225-12-0037 [2012])FDA and the National Institutes of Health (NIH), the Centers for Disease Control and Prevention (CDC), and the Office of the Secretary for Preparedness and Response (ASPR)/Biomedical Advanced Research and Development Authority (BARDA), HHS
May 19, 2016: For development of innovative technologies to identify antimicrobial-resistant bacteria.FDA, the National Institutes of Health (NIH), and the Animal and Plant Health Inspection Service (APHIS), U.S. Department of Agriculture (USDA)
April 29, 2016: Sets forth a framework for reciprocal cooperation which will assist each agency in meeting its responsibilities in promoting proper laboratory animal care and welfare.FDA and the Centers for Disease Control and Prevention (CDC) [MOU 225-16-008]
February 19, 2016: Establishes a framework for the agencies’ coordination in support of CDC’s use of its delegated authority to develop and issue emergency use instructions (EUI) for eligible medical countermeasures.FDA and the HHS Public Health Emergency Medical Countermeasures Enterprise (PHEMCE) [MOU 225-13-0013]
March 11, 2014: Establishes a framework to promote efficiency and collaboration between FDA and PHEMCE partners to meet needs for issues related to safety, efficacy and use of drugs, biologics and medical devices for use in emergencies.FDA and the HHS Office of the Assistant Secretary Secretary for Preparedness and Response (ASPR) [MOU 225-12-0015]
March 21, 2012: Promotes collaboration and enhances knowledge and efficiency by providing for the sharing of information and expertise between Federal partners.
International Confidentiality Commitments - Current and Potential Public Health Emergencies
FDA and the World Health Organization Department of Essential Medicines and Health Products (WHO EMP)
August 25, 2014: To help facilitate communications regarding an actual or potential public health (PH) crisis or PH emergency of international concern; allows sharing of information that is non-public but important to address public health emergencies.
International Confidentiality Commitments - 2014-2015 Ebola Epidemic in West Africa
FDA and the Ministry of Public Health and Hygiene of Guinea (PDF - 364KB)
September 1, 2015: To help facilitate communications between the two agencies on medical products used, or proposed to be used, for Ebola-related purposes as part of cooperative regulatory activities.FDA and the Pharmacy Board of Sierra Leone (PBSL) (PDF - 702KB)
March 18, 2015: To help facilitate communications between FDA and the PBSL on medical products used, or proposed to be used, for Ebola-related purposes as part of cooperative regulatory activities.FDA and the Liberian Medicines and Health Products Regulatory Authority (LMHRA) (PDF - 821KB)
February 3, 2015: To help facilitate communications between FDA and the LMHRA on medical products used, or proposed to be used, for Ebola-related purposes as part of cooperative regulatory activities.
Other International Arrangements - 2014-2015 Ebola Epidemic in West Africa
International Coalition of Medicines Regulatory Authorities (ICMRA) - Statement
September 4, 2014: Medicines regulators to work together internationally to find innovative solutions to facilitate evaluation of and access to potential new medicines to counter Ebola outbreaks
International Confidentiality Commitments - Middle East Respiratory Syndrome Coronavirus
FDA and the Saudi Food and Drug Authority (SFDA) (PDF - 185KB)
October 20, 2015: To help facilitate communications between the two agencies on medical products used, or proposed to be used, for Middle East Respiratory Syndrome Coronavirus (MERS-CoV) as part of cooperative regulatory activities.
Joint Statement of Continued Cooperation Between FDA and ANVISA, Zika Virus Disease
FDA and the Brazilian Health Regulatory Agency (ANVISA) [ARCHIVED]
April 11, 2016: Statement of Continued Cooperation Between FDA and ANVISA - Zika Virus DiseaseFDA ea Agência Nacional de Saúde (ANVISA)[ARCHIVED]
11 de abril de 2016: Declaração conjunta FDA- ANVISA Termo de Cooperação Contínua entre ANVISA e FDA - Vírus Zika
More in MCM Legal, Regulatory and Policy Framework
MCM-Related Legal and Policy Presentations, Publications and Q&As;MCM Emergency Use AuthoritiesMCM-Related Counterterrorism LegislationState, Tribal, Local, and Territorial Public Health PreparednessGuidances and Other Information of Special Interest to MCM StakeholdersMCM-Related Cooperative Arrangements
More relevant information
Another View about Safe and Effective of Bioagents
Pharmaceutical cGMPS for the 21st Century
4,000 Patents: Why the ‘Novel’ SARS-CoV-2 Virus Isn’t so Novel
WHO Regulation and Prequalification
Network of the National Competent Authorities
Carter H, Drury J, Rubin GJ, Williams R, Amlôt R. Applying crowd psychology to develop recommendations for the management of mass decontamination. Health security. 2015;13(1):45-53.
https://history.nih.gov/display/history/Nuremberg+Code
The Blind Spot in COVID-19 Vaccination Policies: Under-Reported Adverse Events
https://ijvtpr.com/index.php/IJVTPR/article/view/65
[1] The Harm Caused by Masks | City Journal (city-journal.org)
[4] https://www.psmid.org/philippine-covid-19-living-recommendations-3/
[6] Pfizer Reports Archives - DailyClout
A Partial Answer to the Question Posed by David A. Hughes, PhD, in the Article: “What is in the so-called COVID-19 ‘Vaccines’? Part 1: Evidence of a Global Crime Against Humanity”
Apparent Cytotoxicity and Intrinsic Cytotoxicity of Lipid Nanomaterials Contained in a COVID-19 mRNA Vaccine
DOI: https://doi.org/10.56098/ijvtpr.v3i1.84
https://rumble.com/v3j3il2-cancer-causing-genes-were-intentionally-used-in-the-covid-death-jabs.html
https://odysee.com/@Liberals_Get_the_Bullet_Too:2/the-next-pandemic:1
https://julimination.wordpress.com/2024/01/28/follow-the-evidence/
https://julimination.wordpress.com/2024/05/14/this-is-not-a-vaccine-by-definition/
https://julimination.wordpress.com/2024/09/30/the-real-goal-of-the-pandemic/
https://julimination.wordpress.com/2024/09/26/omim-online-mendelian-inheritance-in-man/
https://julimination.wordpress.com/2024/09/28/covid-cg-covid-19-cov-genetics-gisaid-who/
The weaponization of artificial intelligence: What the public needs to be aware of
o journalism again & hold Trump to the same standards they hold everyone else to?
Last Wednesday, U.S. Attorney General William Barr
THE PRE-CRIME DRAGNET TAKES SHAPE
More recently, Barr and U.K. Home Secretary Priti Patel
SLEEPWALKING INTO A NIGHTMARE
This widely overlooked background is crucial to understanding William Barr’s recent memorandum and the massive and greatly underreported shift in the policy it heralds. Over a period of several months, Barr — aided by “private sector partners” as well as other current and former government officials — has been laying the groundwork for the system he has now formally announced.
NO BRAINWASHING FOR PRESIDENTS
Mirrored From:
U.S. Military’s Operation Warp Speed Tracking System (3 Whitney Webb articles and 4 videos)
Source: Bullet Proof .Net
Military’s Operation Warp Speed Tracking System
Whitney Webb: “There is a new push to create anational, wastewater surveillance system underground by HHS (Health and Human Services). The system is calledHHS Protect Public Data Hub. This is the data warehouse where all Covid-19 data in country is being housed and is being run byPalantir, which was set up by Peter Thiel and CIA.
PUBLISHED NOVEMBER 21, 2020
Was set up by the CIA’s INQ-TEL, and was successor Chiliad (which set up by Christine Maxwell (Ghislaine’s sister) to handle War on Terror data and Alan Wade (CIA who was also involved in Total Information Awareness Program after 9/11 and in advising Palantir’s product development). This data is highly secret and is being hidden from health officials. It is being used to predict coronavirus outbreaks before any infections occur. Is hidden from local health officials.
OperationWarpSpeedis Using a CIA-Linked Contractor to Keep Covid-19 Vaccine Contracts Secret
Operation Warp Speed: The Role of the US DoD and Partners in the CV19 War on Humanity
Sasha Latypova
https://www.wikiwand.com/en/Total_Information_Awareness
LET’S KEEP IT SHORT BY SIMPLY ANSWERING THESE KEY QUESTIONS:
Can you list the top facts and stats about Total Information Awareness?
Total Information Awareness
The Logo That Took Down a DARPA Surveillance Project
Some of the military-technology agency’s images are disconcerting. Others are actually kind of cute.By
Sunday, March 25, 2012
Total Information Awareness
Have you ever read George Orwell’s
A Dehomag IBM poster, circa 1934. Approximate English translation: “See everything with Hollerith punch cards.” (photo credit: courtesy/Edwin Black
https://www.timesofisrael.com/ibm-and-the-holocaust-by-edwin-black/
How Elon Musk Was Trained in Psychological Manipulation via a Jeffrey Epstein-Funded Program.
In 2011, a small group of extremely influential people met to discuss the future of humanity. Among their ranks were Elon Musk, Jeff Bezos, Nathan Myhrvold, Sergey Brin, and the infamous pedophile child trafficker, Jeffrey Epstein. But this was not the first time these powerful and affluent technophiles had met to determine how best to control society.
Read the article at https://newspaste.com/2024/10/31/musk-epstein-the-third-culture-dossier/
The UN, Corruption and Scandals: How Did We Get Here?
Follow the money, the patents and the evidence!
"We Robot" Elon Musk is a Key Player in the Technocracy Beast System. Rebel Call
Interview On Truth Be Told With Todd Callender. Esq - TransHuman
https://julimination.wordpress.com/tag/have-you-ever-heard-about-the-fraud-of-abdahaldens-enzymes/
https://julimination.wordpress.com/tag/healthy-markets-for-drug-dealers-eugenicists/
https://julimination.wordpress.com/2024/08/24/mathematical-population-genetics-1n/
https://julimination.wordpress.com/2024/08/20/biowarfare-bioethics-and-the-holocaust/
https://julimination.wordpress.com/2024/08/19/german-doctors-and-the-final-solution-2/














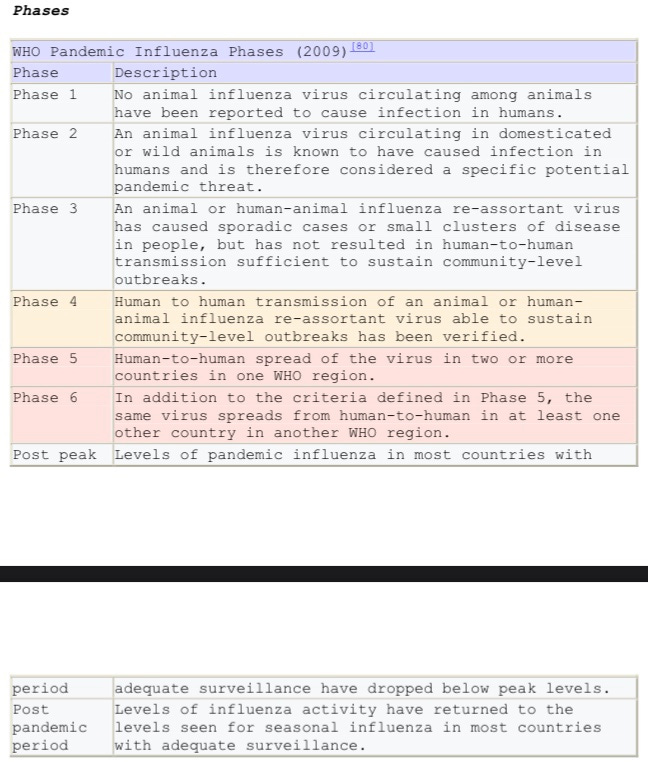






















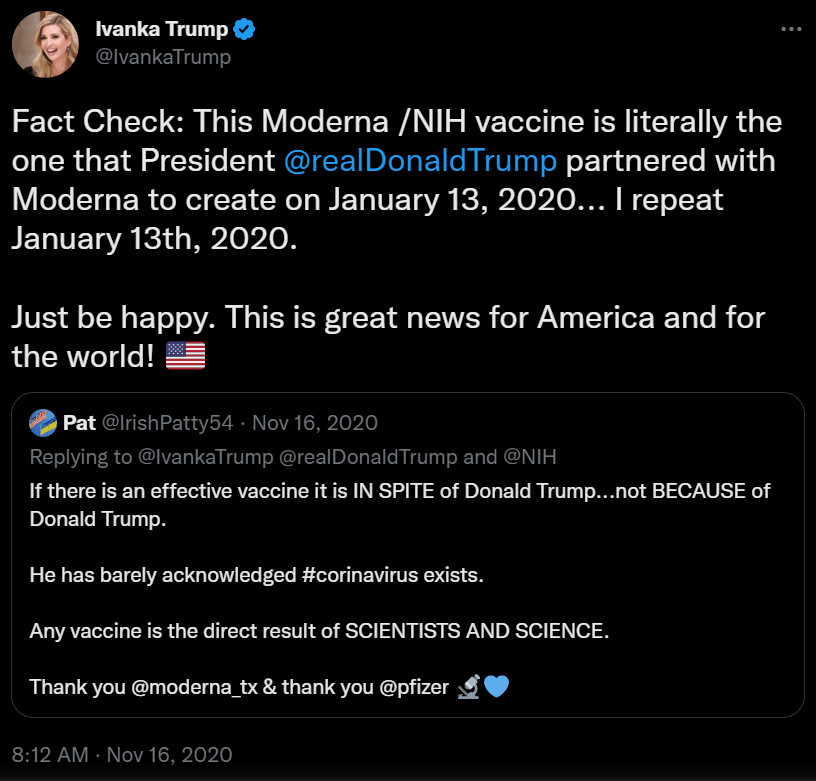







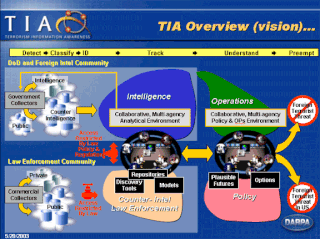



Great article - I can see a great deal of work went into it and I applaud you for it.
BUT - There is a major event that you forgot to include. An event that changed the world as we know it. An attack on human consciousness, a trauma based mind control event that set the stage for people to outsource their thinking, once and for all.....
But also keep in mind, science died on April 23rd, 1984 - David Rasnick
David Rasnick is a biochemist who has spent decades studying AIDS and cancer.
He created DATE analysis as part of his cancer theory work. (DATE stands for Differentiation, adaptation, Transformation, Evolution.)
He knows what he is talking about, in other words.
David Rasnick on the AIDS hoax - https://jermwarfare.com/conversations/david-rasnick-hiv-aids
Not just AIDS had people fooled, but there was A MAJOR cover-up by the power industry, that directly aligns with the seminal event you did not include in your article and if that event was properly understood by the 1st emergent of a "truth" movement, then maybe CV-19 would have never happened and our world would be the exact opposite of what it is now.
We need to rewind 23 years...
You need to revisit 9/11 and the COINTELPRO infiltrated 9/11 "truth" movement and their lies people still cannot break free of.
You already see how many people are still diaper wearing, vaccine believing sheep...
You need to investigate "cold fusion" and let me help you on your journey...
Cold Fusion: Fire from Water (1998) - Cold fusion, one of the most important scientific discoveries in history, burst onto the world scene in 1989. This much maligned yet now confirmed discovery may radically change the energy structure of civilization.
Video: https://rumble.com/v5lnot6-cold-fusion-fire-from-water-1998.html
"Cold Fusion", Steven E. Jones, Thermite & 9/11 - Contrary to the "nanothermite" hypothesis of the "Architects and Engineers for 9/11 truth," the Twin Towers were evidently destroyed at low temperatures, revealing the reality of "Cold Fusion". - Prof David A, Hughes
Video: https://rumble.com/v5lto5w-cold-fusion-steven-e.-jones-thermite-and-911.html
9/11 Truth Suppression Timeline
"The best way to control the opposition is to lead it ourselves." - Vladimir Lenin
Article: https://911revision.substack.com/p/911-truth-suppression-timeline
9/11 Evidence presented by Dr Judy Wood – Prof David A, Hughes
Contrary to the "nanothermite" hypothesis of the "Architects and Engineers for 9/11 truth," the Twin Towers were evidently destroyed at low temperatures, revealing the reality of "Cold Fusion".
Article: https://dhughes.substack.com/p/in-defence-of-judy-wood-0ce
with regards to the technology used on 9/11, MOST are missing the bigger picture!
Just as the hazardous and wasteful technology behind a nuclear bomb can also be used to provide hazardous and wasteful nuclear power, the technology which caused the clean and effortless molecular dissociation of the twin towers could also be used to give the whole world effortless clean energy.
Exposing this clean free energy technology means and end to the ruling elite's ability to control and exploit the general population through scarce, expensive, dirty and inefficient resources such as oil, coal, nuclear and "renewables".
Any group with an interest in maintaining the current paradigm of artificial scarcity and crappy energy technologies, which keeps the general population enslaved, would have an interest in helping to maintain the 9/11 cover up, because exposing the crime also exposes the TECHNOLOGY to commit it.
Maybe the people who did 9/11 operate under a code of conduct where they have to show this technology to the people, so they have the opportunity to claim it.
And if the people are too stupid or apathetic to show any interest, they will then feel justified in keeping it for themselves and continuing to control and exploit the human herd, like the CATTLE they have shown themselves to be.
You might think it's insane, and it is, but remember we're talking about a group of people who are willing to turn skyscrapers to DUST, live on TV.
In the final analysis there is no "grand" deception" or cover up. There is only BLUFF!
Because everyone can see what happened to the buildings was clearly and PROVABLY NOT a structural failure (collapse) or a conventional controlled demolition by ANY thermal or kinetic mechanism!
Official narrative – Jet fuel.
Option behind door no 1 – explosives,
door no 2 – thermite,
door no 3 – buried or mini nukes.
Just don’t look at where the EVIDENCE points to, a black-ops cold DEW technology that can direct energy to disrupt the molecular bonds of matter. (Direct or control – where it goes and what it does)
Read Dr Wood’s book: Where did the towers go? https://www.wheredidthetowersgo.com
Andrew Johnson's two FREE E-Books on 9/11:
1. 9/11 – Finding the Truth - http://www.checktheevidence.co.uk/pdf/9-11%20-%20Finding%20the%20Truth.pdf
2. 9/11 – Holding the Truth - http://checktheevidencecom.ipage.com/checktheevidence.com/pdf/911%20Holding%20The%20Truth%20-Andrew%20Johnson%20-%202017.pdf